Regular cannabis use alters the neural dynamics serving complex motor control
- PMID: 37955378
- PMCID: PMC10681654
- DOI: 10.1002/hbm.26527
Regular cannabis use alters the neural dynamics serving complex motor control
Abstract
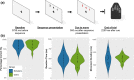
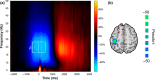
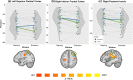
Cannabis is the most widely used recreational drug in the United States and regular use has been linked to deficits in attention and memory. However, the effects of regular use on motor control are less understood, with some studies showing deficits and others indicating normal performance. Eighteen users and 23 nonusers performed a motor sequencing task during high-density magnetoencephalography (MEG). The MEG data was transformed into the time-frequency domain and beta responses (16-24 Hz) during motor planning and execution phases were imaged separately using a beamformer approach. Whole-brain maps were examined for group (cannabis user/nonuser) and time window (planning/execution) effects. As expected, there were no group differences in task performance (e.g., reaction time, accuracy, etc.). Regular cannabis users exhibited stronger beta oscillations in the contralateral primary motor cortex compared to nonusers during the execution phase of the motor sequences, but not during the motor planning phase. Similar group-by-time window interactions were observed in the left superior parietal, right inferior frontal cortices, right posterior insular cortex, and the bilateral motor cortex. We observed differences in the neural dynamics serving motor control in regular cannabis users compared to nonusers, suggesting regular users may employ compensatory processing in both primary motor and higher-order motor cortices to maintain adequate task performance. Future studies will need to examine more complex motor control tasks to ascertain whether this putative compensatory activity eventually becomes exhausted and behavioral differences emerge.
Keywords: beta; gamma; marijuana; motor sequencing; movement; oscillations.
© 2023 The Authors. Human Brain Mapping published by Wiley Periodicals LLC.
Conflict of interest statement
The authors of this manuscript acknowledge no conflicts of interest, financial or otherwise.
Figures

References
-
- Arpin, D. J. , Heinrichs‐Graham, E. , Gehringer, J. E. , Zabad, R. , Wilson, T. W. , & Kurz, M. J. (2017). Altered sensorimotor cortical oscillations in individuals with multiple sclerosis suggests a faulty internal model. Human Brain Mapping, 38(8), 4009–4018. 10.1002/hbm.23644 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
- F31 DA056296/DA/NIDA NIH HHS/United States
- R36-DA059323/NH/NIH HHS/United States
- R36 DA059323/DA/NIDA NIH HHS/United States
- R03-DA041917/NH/NIH HHS/United States
- P20 GM144641/GM/NIGMS NIH HHS/United States
- R01 DA047828/DA/NIDA NIH HHS/United States
- R03 DA041917/DA/NIDA NIH HHS/United States
- R01-MH116782/NH/NIH HHS/United States
- R01-MH103220/NH/NIH HHS/United States
- F31-DA056296/NH/NIH HHS/United States
- R01-MH118013/NH/NIH HHS/United States
- R01-DA056223/NH/NIH HHS/United States
- F30 AG076259/AG/NIA NIH HHS/United States
- P20-GM144641/NH/NIH HHS/United States
- F30 MH130150/MH/NIMH NIH HHS/United States
- R01 DA056223/DA/NIDA NIH HHS/United States
- R01 MH103220/MH/NIMH NIH HHS/United States
- R01 MH118013/MH/NIMH NIH HHS/United States
- R01-DA047828/NH/NIH HHS/United States
- R01 MH116782/MH/NIMH NIH HHS/United States
- F30-MH130150/NH/NIH HHS/United States
- F30-AG076259/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

