Quantitative Gait and Balance Outcomes for Ataxia Trials: Consensus Recommendations by the Ataxia Global Initiative Working Group on Digital-Motor Biomarkers
- PMID: 37955812
- PMCID: PMC11269489
- DOI: 10.1007/s12311-023-01625-2
Quantitative Gait and Balance Outcomes for Ataxia Trials: Consensus Recommendations by the Ataxia Global Initiative Working Group on Digital-Motor Biomarkers
Abstract
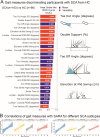
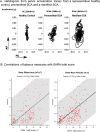
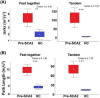
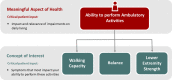
With disease-modifying drugs on the horizon for degenerative ataxias, ecologically valid, finely granulated, digital health measures are highly warranted to augment clinical and patient-reported outcome measures. Gait and balance disturbances most often present as the first signs of degenerative cerebellar ataxia and are the most reported disabling features in disease progression. Thus, digital gait and balance measures constitute promising and relevant performance outcomes for clinical trials.This narrative review with embedded consensus will describe evidence for the sensitivity of digital gait and balance measures for evaluating ataxia severity and progression, propose a consensus protocol for establishing gait and balance metrics in natural history studies and clinical trials, and discuss relevant issues for their use as performance outcomes.
Keywords: Cerebellar ataxia; Digital motor performance marker; Gait and posture.
© 2023. The Author(s).
Conflict of interest statement
Fay Horak and Vrutangkumar Shah and James McNames are employees of APDM Precision Motion, Clario, a company that may have a commercial interest in the results of this research and technology. This potential conflict of interest has been reviewed and managed by OHSU.
Winfried Ilg receives consultancy honoraria from Ionis Pharmaceuticals.
Enrico Bertini receives honoraria for Advisory Board from PTC Therapeutics, Biogen, Roche.
Christopher Gomez discloses research collaboration with APDM Precision Motion, Clario.
Tanja Schmitz-Hübsch discloses research collaboration with a provider of a motion capture system based on Kinect technology (Motognosis GmbH, Berlin, Germany).
Helen Dawes is Director of International Affairs Physiobiometrics inc and scientific advisor for Elaros, UK.
Kirsi M Kinnunen ist employed by IXICO, London, UK
Hasmet Hanagasi, Jane Newman, Sarah Milne, Clara Rentz, Lukas Beichert, Yi Ng, Lisa Alcock, Norlinah Mohamed Ibrahim, Martina Minneropt, Andrea H Nemeth, Bedia Samanci, Vrutangkumar Shah, Susanna Summa, Gessica Vasco report no disclosures.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

