Bayesian spatial modelling of localised SARS-CoV-2 transmission through mobility networks across England
- PMID: 37956206
- PMCID: PMC10756685
- DOI: 10.1371/journal.pcbi.1011580
Bayesian spatial modelling of localised SARS-CoV-2 transmission through mobility networks across England
Abstract
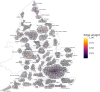
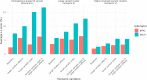
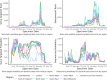
In the early phases of growth, resurgent epidemic waves of SARS-CoV-2 incidence have been characterised by localised outbreaks. Therefore, understanding the geographic dispersion of emerging variants at the start of an outbreak is key for situational public health awareness. Using telecoms data, we derived mobility networks describing the movement patterns between local authorities in England, which we have used to inform the spatial structure of a Bayesian BYM2 model. Surge testing interventions can result in spatio-temporal sampling bias, and we account for this by extending the BYM2 model to include a random effect for each timepoint in a given area. Simulated-scenario modelling and real-world analyses of each variant that became dominant in England were conducted using our BYM2 model at local authority level in England. Simulated datasets were created using a stochastic metapopulation model, with the transmission rates between different areas parameterised using telecoms mobility data. Different scenarios were constructed to reproduce real-world spatial dispersion patterns that could prove challenging to inference, and we used these scenarios to understand the performance characteristics of the BYM2 model. The model performed better than unadjusted test positivity in all the simulation-scenarios, and in particular when sample sizes were small, or data was missing for geographical areas. Through the analyses of emerging variant transmission across England, we found a reduction in the early growth phase geographic clustering of later dominant variants as England became more interconnected from early 2022 and public health interventions were reduced. We have also shown the recent increased geographic spread and dominance of variants with similar mutations in the receptor binding domain, which may be indicative of convergent evolution of SARS-CoV-2 variants.
Copyright: © 2023 Ward et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
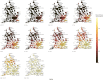


References
-
- UKHSA. What Does COVID-19 look like in your area? Available from: https://ukhsa.blog.gov.uk/2021/12/16/what-does-covid-19-look-like-in-you...; 2021.
-
- UKHSA. Weekly statistics for rapid asymptomatic testing (England). Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploa...; 2021.
-
- Department of Health and Social Care. Everyone in the United Kingdom with symptoms now eligible for coronavirus tests. Available from: https://www.gov.uk/government/news/everyone-in-the-united-kingdom-with-s...; 2020.
-
- Department of Health and Social Care. Health Secretary launches biggest diagnostic lab network in British history to test for coronavirus. Available from: https://www.gov.uk/government/news/health-secretary-launches-biggest-dia...; 2020.
-
- Green MA, García-Fiñana M, Barr B, Burnside G, Cheyne CP, Hughes D et al.. Evaluating social and spatial inequalities of large scale rapid lateral flow SARS-CoV-2 antigen testing in COVID-19 management: an observational study of Liverpool, UK (November 2020 to January 2021). Lancet Reg Health Eur. 2021;6: 100107. doi: 10.1016/j.lanepe.2021.100107 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

