A B cell-driven EAE mouse model reveals the impact of B cell-derived cytokines on CNS autoimmunity
- PMID: 37956299
- PMCID: PMC10666104
- DOI: 10.1073/pnas.2300733120
A B cell-driven EAE mouse model reveals the impact of B cell-derived cytokines on CNS autoimmunity
Abstract
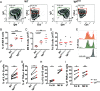
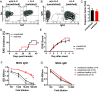
In multiple sclerosis (MS), pathogenic T cell responses are known to be important drivers of autoimmune inflammation. However, increasing evidence suggests an additional role for B cells, which may contribute to pathogenesis via antigen presentation and production of proinflammatory cytokines. However, these B cell effector functions are not featured well in classical experimental autoimmune encephalomyelitis (EAE) mouse models. Here, we compared properties of myelin oligodendrocyte glycoprotein (MOG)-specific and polyclonal B cells and developed an adjuvant-free cotransfer EAE mouse model, where highly activated, MOG-specific induced germinal center B cells provide the critical stimulus for disease development. We could show that high levels of MOG-specific immunoglobulin G (IgGs) are not required for EAE development, suggesting that antigen presentation and activation of cognate T cells by B cells may be important for pathogenesis. As our model allows for B cell manipulation prior to transfer, we found that overexpression of the proinflammatory cytokine interleukin (IL)-6 by MOG-specific B cells leads to an accelerated EAE onset accompanied by activation/expansion of the myeloid compartment rather than a changed T cell response. Accordingly, knocking out IL-6 or tumor necrosis factor α in MOG-specific B cells via CRISPR-Cas9 did not affect activation of pathogenic T cells. In summary, we generated a tool to dissect pathogenic B cell effector function in EAE development, which should improve our understanding of pathogenic processes in MS.
Keywords: B cells; CNS autoimmunity; EAE/MS; cytokines.
Conflict of interest statement
The authors declare no competing interest.
Figures



References
-
- Magliozzi R., et al. , Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain. 130, 1089–1104 (2007). - PubMed
-
- Mader S., Kümpfel T., Meinl E., Novel insights into pathophysiology and therapeutic possibilities reveal further differences between AQP4-IgG- and MOG-IgG-associated diseases. Curr. Opin. Neurol. 33, 362–371 (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

