An umbrella review of candidate predictors of response, remission, recovery, and relapse across mental disorders
- PMID: 37957292
- PMCID: PMC10730397
- DOI: 10.1038/s41380-023-02298-3
An umbrella review of candidate predictors of response, remission, recovery, and relapse across mental disorders
Abstract
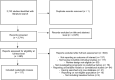
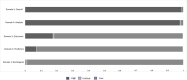
We aimed to identify diagnosis-specific/transdiagnostic/transoutcome multivariable candidate predictors (MCPs) of key outcomes in mental disorders. We conducted an umbrella review (protocol link ), searching MEDLINE/Embase (19/07/2022), including systematic reviews of studies reporting on MCPs of response, remission, recovery, or relapse, in DSM/ICD-defined mental disorders. From published predictors, we filtered MCPs, validating MCP criteria. AMSTAR2/PROBAST measured quality/risk of bias of systematic reviews/individual studies. We included 117 systematic reviews, 403 studies, 299,888 individuals with mental disorders, testing 796 prediction models. Only 4.3%/1.2% of the systematic reviews/individual studies were at low risk of bias. The most frequently targeted outcome was remission (36.9%), the least frequent was recovery (2.5%). Studies mainly focused on depressive (39.4%), substance-use (17.9%), and schizophrenia-spectrum (11.9%) disorders. We identified numerous MCPs within disorders for response, remission and relapse, but none for recovery. Transdiagnostic MCPs of remission included lower disease-specific symptoms (disorders = 5), female sex/higher education (disorders = 3), and quality of life/functioning (disorders = 2). Transdiagnostic MCPs of relapse included higher disease-specific symptoms (disorders = 5), higher depressive symptoms (disorders = 3), and younger age/higher anxiety symptoms/global illness severity/ number of previous episodes/negative life events (disorders = 2). Finally, positive trans-outcome MCPs for depression included less negative life events/depressive symptoms (response, remission, less relapse), female sex (response, remission) and better functioning (response, less relapse); for schizophrenia, less positive symptoms/higher depressive symptoms (remission, less relapse); for substance use disorder, marital status/higher education (remission, less relapse). Male sex, younger age, more clinical symptoms and comorbid mental/physical symptoms/disorders were poor prognostic factors, while positive factors included social contacts and employment, absent negative life events, higher education, early access/intervention, lower disease-specific and comorbid mental and physical symptoms/conditions, across mental disorders. Current data limitations include high risk of bias of studies and extraction of single predictors from multivariable models. Identified MCPs can inform future development, validation or refinement of prediction models of key outcomes in mental disorders.
© 2023. The Author(s).
Conflict of interest statement
CUC has been a consultant and/or advisor to or has received honoraria from: AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Boehringer-Ingelheim, Cardio Diagnostics, Cerevel, CNX Therapeutics, Compass Pathways, Darnitsa, Denovo, Gedeon Richter, Hikma, Holmusk, IntraCellular Therapies, Janssen/J&J, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedInCell, Merck, Mindpax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Newron, Noven, Novo Nordisk, Otsuka, Pharmabrain, PPD Biotech, Recordati, Relmada, Reviva, Rovi, Seqirus, SK Life Science, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Compass Pathways, Denovo, Lundbeck, Relmada, Reviva, Rovi, Supernus, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, Mindpax, LB Pharma and Quantic. CR received the 2020 Lundbeck Foundation Talent Prize. OK-F received honoraria for lectures for Lundbeck Pharma A/S and consultant work for WCG Clinical. MH has been consultant for or has received honoraria from: H. Lundbeck, The Lundbeck Foundation and Otsuka. MS received honoraria/has been a consultant for AbbVie, Angelini, Lundbeck, Otsuka. All other authors declare no conflict of interest.
Figures
References
-
- Solmi M, Taipale H, Holm M, Tanskanen A, Mittendorfer-Rutz E, Correll CU, et al. Effectiveness of antipsychotic use for reducing risk of work disability: results from a within-subject analysis of a Swedish National Cohort of 21,551 patients with first-episode nonaffective psychosis. Am J Psychiatry. 2022;179:938–46. - PubMed
-
- Leucht S, Leucht C, Huhn M, Chaimani A, Mavridis D, Helfer B, et al. Sixty years of placebo-controlled antipsychotic drug trials in acute schizophrenia: systematic review, bayesian meta-analysis, and meta-regression of efficacy predictors. Am J Psychiatry. 2017;174:927–42. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

