Chia seeds and coenzyme Q10 alleviate iron overload induced hepatorenal toxicity in mice via iron chelation and oxidative stress modulation
- PMID: 37957293
- PMCID: PMC10643458
- DOI: 10.1038/s41598-023-47127-3
Chia seeds and coenzyme Q10 alleviate iron overload induced hepatorenal toxicity in mice via iron chelation and oxidative stress modulation
Abstract
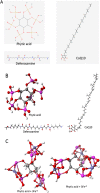
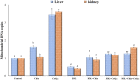
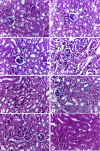
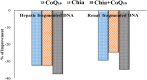
Iron overload (IOL) can cause hepatorenal damage due to iron-mediated oxidative and mitochondrial damage. Remarkably, combining a natural iron chelator with an antioxidant can exert greater efficacy than monotherapy. Thus, the present study aimed to evaluate the efficacy of Chia and CoQ10 to chelate excess iron and prevent hepatorenal oxidative damage in IOL mice. Male Swiss albino mice (n = 49) were randomly assigned to seven groups: control, dietary Chia, CoQ10, IOL, IOL + Chia, IOL + CoQ10, and IOL + Chia + CoQ10. Computational chemistry indicates that the phytic acid found in the Chia seeds is stable, reactive, and able to bind to up to three iron ions (both Fe2+ and Fe3+). IOL induced a significant (P < 0.05) increase in serum iron, ferritin, transferrin, TIBC, TSI, RBCs, Hb, MCV, MCH, WBCs, AST, ALT, creatinine, and MDA. IOL causes a significant (P < 0.05) decrease in UIBC, platelets, and antioxidant molecules (GSH, SOD, CAT, and GR). Also, IOL elicits mitochondrial membrane change depolarization, and DNA fragmentation and suppresses mitochondrial DNA copies. Furthermore, substantial changes in hepatic and renal tissue, including hepatocellular necrosis and apoptosis, glomerular degeneration, glomerular basement membrane thickening, and tubular degeneration, were observed in the IOL group. Dietary Chia and CoQ10 induced significant (P < 0.05) amelioration in all the mentioned parameters. They can mostly repair the abnormal architecture of hepatic and renal tissues induced by IOL, as signified by normal sinusoids, normal central veins, and neither glomerular damage nor degenerated tubules. In conclusion, the combined treatment with Chia + CoQ10 exerts more pronounced efficacy than monotherapy in hepatorenal protection via chelating excess iron and improved cellular antioxidant status and hepatorenal mitochondrial function in IOL mice.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

