PROACTING: predicting pathological complete response to neoadjuvant chemotherapy in breast cancer from routine diagnostic histopathology biopsies with deep learning
- PMID: 37957667
- PMCID: PMC10644597
- DOI: 10.1186/s13058-023-01726-0
PROACTING: predicting pathological complete response to neoadjuvant chemotherapy in breast cancer from routine diagnostic histopathology biopsies with deep learning
Abstract
Background: Invasive breast cancer patients are increasingly being treated with neoadjuvant chemotherapy; however, only a fraction of the patients respond to it completely. To prevent overtreatment, there is an urgent need for biomarkers to predict treatment response before administering the therapy.
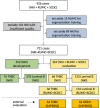
Methods: In this retrospective study, we developed hypothesis-driven interpretable biomarkers based on deep learning, to predict the pathological complete response (pCR, i.e., the absence of tumor cells in the surgical resection specimens) to neoadjuvant chemotherapy solely using digital pathology H&E images of pre-treatment breast biopsies. Our approach consists of two steps: First, we use deep learning to characterize aspects of the tumor micro-environment by detecting mitoses and segmenting tissue into several morphology compartments including tumor, lymphocytes and stroma. Second, we derive computational biomarkers from the segmentation and detection output to encode slide-level relationships of components of the tumor microenvironment, such as tumor and mitoses, stroma, and tumor infiltrating lymphocytes (TILs).
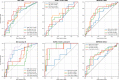
Results: We developed and evaluated our method on slides from n = 721 patients from three European medical centers with triple-negative and Luminal B breast cancers and performed external independent validation on n = 126 patients from a public dataset. We report the predictive value of the investigated biomarkers for predicting pCR with areas under the receiver operating characteristic curve between 0.66 and 0.88 across the tested cohorts.
Conclusion: The proposed computational biomarkers predict pCR, but will require more evaluation and finetuning for clinical application. Our results further corroborate the potential role of deep learning to automate TILs quantification, and their predictive value in breast cancer neoadjuvant treatment planning, along with automated mitoses quantification. We made our method publicly available to extract segmentation-based biomarkers for research purposes.
Keywords: Computational biomarker; Neoadjuvant chemotherapy; Pathological complete response.
© 2023. The Author(s).
Conflict of interest statement
FC was Chair of the Scientific and Medical Advisory Board of TRIBVN Healthcare, France, and received advisory board fees from TRIBVN Healthcare, France, in the last five years. He is shareholder of Aiosyn BV, the Netherlands. MB is medical advisor at Aiosyn BV. All other authors declare no conflict of interest. JvdL was a member of the advisory boards of Philips, the Netherlands and ContextVision, Sweden, and received research funding from Philips, the Netherlands, ContextVision, Sweden, and Sectra, Sweden in the last five years. He is chief scientific officer (CSO) and shareholder of Aiosyn BV, the Netherlands.
Figures



References
-
- Gamucci T, Pizzuti L, Sperduti I, Mentuccia L, Vaccaro A, Moscetti L, Marchetti P, Carbognin L, Michelotti A, Iezzi L, et al. Neoadjuvant chemotherapy in triple-negative breast cancer: a multicentric retrospective observational study in real-life setting. J Cell Physiol. 2018;233(3):2313–2323. doi: 10.1002/jcp.26103. - DOI - PubMed
-
- Bonnefoi H, Litière S, Piccart M, MacGrogan G, Fumoleau P, Brain E, Petit T, Rouanet P, Jassem J, Moldovan C, et al. Pathological complete response after neoadjuvant chemotherapy is an independent predictive factor irrespective of simplified breast cancer intrinsic subtypes: a landmark and two-step approach analyses from the eortc 10994/big 1–00 phase iii trial. Ann Oncol. 2014;25(6):1128–1136. doi: 10.1093/annonc/mdu118. - DOI - PMC - PubMed
-
- Denkert C, Loibl S, Noske A, Roller M, Muller B, Komor M, Budczies J, Darb-Esfahani S, Kronenwett R, Hanusch C, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

