Treatment with recombinant Sirt1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy
- PMID: 37957697
- PMCID: PMC10644415
- DOI: 10.1186/s12933-023-02057-2
Treatment with recombinant Sirt1 rewires the cardiac lipidome and rescues diabetes-related metabolic cardiomyopathy
Abstract
Background: Metabolic cardiomyopathy (MCM), characterized by intramyocardial lipid accumulation, drives the progression to heart failure with preserved ejection fraction (HFpEF). Although evidence suggests that the mammalian silent information regulator 1 (Sirt1) orchestrates myocardial lipid metabolism, it is unknown whether its exogenous administration could avoid MCM onset. We investigated whether chronic treatment with recombinant Sirt1 (rSirt1) could halt MCM progression.
Methods: db/db mice, an established model of MCM, were supplemented with intraperitoneal rSirt1 or vehicle for 4 weeks and compared with their db/ + heterozygous littermates. At the end of treatment, cardiac function was assessed by cardiac ultrasound and left ventricular samples were collected and processed for molecular analysis. Transcriptional changes were evaluated using a custom PCR array. Lipidomic analysis was performed by mass spectrometry. H9c2 cardiomyocytes exposed to hyperglycaemia and treated with rSirt1 were used as in vitro model of MCM to investigate the ability of rSirt1 to directly target cardiomyocytes and modulate malondialdehyde levels and caspase 3 activity. Myocardial samples from diabetic and nondiabetic patients were analysed to explore Sirt1 expression levels and signaling pathways.
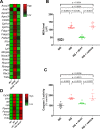
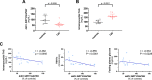
Results: rSirt1 treatment restored cardiac Sirt1 levels and preserved cardiac performance by improving left ventricular ejection fraction, fractional shortening and diastolic function (E/A ratio). In left ventricular samples from rSirt1-treated db/db mice, rSirt1 modulated the cardiac lipidome: medium and long-chain triacylglycerols, long-chain triacylglycerols, and triacylglycerols containing only saturated fatty acids were reduced, while those containing docosahexaenoic acid were increased. Mechanistically, several genes involved in lipid trafficking, metabolism and inflammation, such as Cd36, Acox3, Pparg, Ncoa3, and Ppara were downregulated by rSirt1 both in vitro and in vivo. In humans, reduced cardiac expression levels of Sirt1 were associated with higher intramyocardial triacylglycerols and PPARG-related genes.
Conclusions: In the db/db mouse model of MCM, chronic exogenous rSirt1 supplementation rescued cardiac function. This was associated with a modulation of the myocardial lipidome and a downregulation of genes involved in lipid metabolism, trafficking, inflammation, and PPARG signaling. These findings were confirmed in the human diabetic myocardium. Treatments that increase Sirt1 levels may represent a promising strategy to prevent myocardial lipid abnormalities and MCM development.
Keywords: Cardiometabolic; Diabetes; Lipidome; Metabolic cardiomyopathy; Sirt1; Therapy.
© 2023. The Author(s).
Conflict of interest statement
T.F.L. has outside this work received research and educational grants to the institution Abbott, Amgen, AstraZeneca, Boehringer-Ingelheim, Daichi-Sankyo, Menarini Foundation, Novartis, Novo Nordisk, Roche Diagnostics, Sanofi and Vifor and honoraria from Amgen, Dacadoo, Daichi-Sankyo, Menarini Foundation, Novartis, Novo Nordisk, Philips and Pfizer. F.R. has not received personal payments from pharmaceutical companies or device manufacturers in the last three years (remuneration for the time spent in activities, such as participation as steering committee member of clinical trials and member of the Pfizer Research Award selection committee in Switzerland, were made directly to the University of Zurich). The Department of Cardiology (University Hospital of Zurich/University of Zurich) reports research-, educational- and/or travel grants from Abbott, Amgen, Astra Zeneca, Bayer, Berlin Heart, B. Braun, Biosense Webster, Biosensors Europe AG, Biotronik, BMS, Boehringer Ingelheim, Boston Scientific, Bracco, Cardinal Health Switzerland, Corteria, Daiichi, Diatools AG, Edwards Lifesciences, Guidant Europe NV (BS), Hamilton Health Sciences, Kaneka Corporation, Kantar, Labormedizinisches Zentrum, Medtronic, MSD, Mundipharma Medical Company, Novartis, Novo Nordisk, Orion, Pfizer, Quintiles Switzerland Sarl, Sahajanand IN, Sanofi, Sarstedt AG, Servier, SIS Medical, SSS International Clinical Research, Terumo Deutschland, Trama Solutions, V-Wave, Vascular Medical, Vifor, Wissens Plus, ZOLL. The research and educational grants do not impact F.R.’s personal remuneration. F.P. has received personal fees from Novo Nordisk and is a scientific consultant for Vectura Pharma.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

