Feasibility and safety of integrating mass drug administration for helminth control with seasonal malaria chemoprevention among Senegalese children: a randomized controlled, observer-blind trial
- PMID: 37957702
- PMCID: PMC10642045
- DOI: 10.1186/s12936-023-04784-z
Feasibility and safety of integrating mass drug administration for helminth control with seasonal malaria chemoprevention among Senegalese children: a randomized controlled, observer-blind trial
Abstract
Background: The overlap in the epidemiology of malaria and helminths has been identified as a potential area to exploit for the development of an integrated control strategy that may help to achieve elimination of malaria and helminths. A randomized, controlled, observer-blind trial was conducted to assess the feasibility and safety of combining mass drug administration (MDA) for schistosomiasis and soil transmitted helminths (STH) with seasonal malaria chemoprevention (SMC) among children living in Senegal.
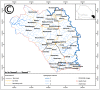
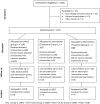
Methods: Female and male children aged 1-14 years were randomized 1:1:1, to receive Vitamin A and Zinc on Day 0, followed by SMC drugs (sulfadoxine-pyrimethamine and amodiaquine) on Days 1-3 (control group); or praziquantel and Vitamin A on Day 0, followed by SMC drugs on Days 1-3 (treatment group 1); or albendazole and praziquantel on Day 0, followed by SMC drugs on Days 1-3 (treatment group 2). Safety assessment was performed by collecting adverse events from all children for six subsequent days following administration of the study drugs. Pre- and post-intervention, blood samples were collected for determination of haemoglobin concentration, malaria microscopy, and PCR assays. Stool samples were analyzed using Kato-Katz, Merthiolate-iodine-formalin and PCR methods. Urine filtration, PCR and circulating cathodic antigen tests were also performed.
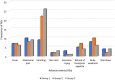
Results: From 9 to 22 June 2022, 627 children aged 1-14 years were randomized into the three groups described above. Mild, transient vomiting was observed in 12.6% (26/206) of children in treatment group 2, in 10.6% (22/207) in group 1, and in 4.2% (9/214) in the control group (p = 0.005). Pre-intervention, the geometric mean value of Plasmodium falciparum parasite density was highest among children who received albendazole, praziquantel with SMC drugs. Post-intervention, the parasite density was highest among children who received SMC drugs only. Children who received praziquantel and SMC drugs had a lower risk of developing severe anaemia than their counterparts who received SMC drugs alone (OR = 0.81, 95% CI 0.13-5.00, p = 0.63).
Conclusions: Integration of MDA for helminths with SMC drugs was safe and feasible among Senegalese children. These findings support further evaluation of the integrated control model.
Trial registration: The study is registered at Clinical Trial.gov NCT05354258.
Keywords: Co-infection; Falciparum malaria; Integrated control strategy; Schistosomiasis; Soil-transmitted helminthiasis.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
-
- WHO . World malaria report 2022. Geneva: World Health Organization; 2022.
-
- WHO . Global report on neglected tropical diseases 2023. Geneva: World Health Organization; 2023.
-
- Kigali Summit on malaria and neglected tropical diseases. https://malariantdsummit.org/. Accessed 05 Sep 2022.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

