Heterogeneity of cortical pTDP-43 inclusion morphologies in amyotrophic lateral sclerosis
- PMID: 37957721
- PMCID: PMC10642010
- DOI: 10.1186/s40478-023-01670-2
Heterogeneity of cortical pTDP-43 inclusion morphologies in amyotrophic lateral sclerosis
Abstract
Background: Despite the presence of significant cortical pTDP-43 inclusions of heterogeneous morphologies in patients diagnosed with amyotrophic lateral sclerosis (ALS), pathological subclassification is routinely performed in the minority of patients with concomitant frontotemporal dementia (FTD).
Objective: In order to improve current understanding of the presence and relevance of pathological pTDP-43 subtypes in ALS, the present study examined the pattern of cortical pTDP-43 aggregates in 61 ALS cases without FTD.
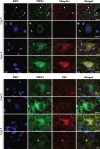
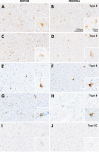
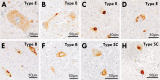
Results: Based on the presence, morphology and composition of pTDP-43 pathology, three distinct ALS-TDP subtypes were delineated: (1) A predominant pattern of pTDP-43 granulofilamentous neuronal inclusions (GFNIs) and grains that were immuno-negative for p62 was identified in 18% of cases designated ALS-TDP type E; (2) neuronal cytoplasmic inclusions (NCIs) that were immuno-positive for both pTDP-43 and p62 were observed in 67% of cases assigned ALS-TDP type B; and (3) scarce cortical pTDP-43 and p62 aggregates were identified in 15% of cases coined ALS-TDP type SC (scarce cortical). Quantitative analyses revealed a significantly greater burden of pTDP-43 GFNI and grains in ALS-TDP type E. Principal component analysis demonstrated significant relationships between GFNIs, grains and ALS-TDP subtypes to support the distinction of subtypes E and B. No significant difference in age at death or disease duration was found between ALS-TDP subgroups to suggest that these subtypes represent earlier or later stages of the same disease process. Instead, a significantly higher ALS-TDP stage, indicating greater topographical spread of pTDP-43, was identified in ALS-TDP type E. Alzheimer's disease neuropathological change (ABC score ≥ intermediate) and Lewy body disease (Braak stage ≥ IV) was more prevalent in the ALS-TDP type SC cohort, which also demonstrated a significantly lower overall cognitive score.
Conclusion: In summary, the present study demonstrates that ALS-TDP does not represent a single homogenous neuropathology. We propose the subclassification of ALS-TDP into three distinct subtypes using standard immuno-stains for pTDP-43 and p62 in the motor cortex, which is routinely sampled and evaluated for diagnostic neuropathological characterisation of ALS. We propose that future studies specify both clinicopathological group and pTDP-43 subtype to advance current understanding of the pathogenesis of clinical phenotypes in pTDP-43 proteinopathies, which will have significant relevance to the development of targeted therapies for this heterogeneous disorder.
Keywords: Amyotrophic lateral sclerosis; Frontotemporal lobar degeneration; pTDP-43.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

