Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents
- PMID: 37958079
- PMCID: PMC10649842
- DOI: 10.3390/ani13213324
Assessing Optimal Cell Counts in Sperm Shape Abnormality Assays in Rodents
Abstract
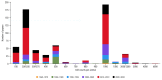
Rodents have been the preferred models for the evaluation of the toxicity of pollutants and drugs and their genotoxic effects, including sperm shape abnormalities. The scientific literature is dominated by studies conducted with model animals in laboratory conditions, but a generally accepted and standardized protocol addressing the optimal number of sperm cells to count is still lacking. In this study, we reviewed the literature regarding the number of counted sperm cells in such assessments, published from 1969 to 2023. To infer the number of counts providing the best cost/benefit regarding the robustness of the assay results, a new dataset involving the analysis of two populations of wild rodents was produced. We evaluated the frequency of sperm shape abnormalities in a total of 50 wild brown rats (Rattus norvegicus) captured in two port cities, aiming to detect the impact of differential sperm cell counts in the obtained results. During necropsy, the fresh epididymis tail of adult male rats was excised, and sperm cells were fixated in slides. For each animal, a total of 300, 500, 1000, and 2000 cells were sequentially counted, and head abnormalities were registered. Counting 300 sperm cells failed to detect significant differences between groups and 500 counts resulted in marginally significant differences. Only when 1000 or 2000 sperm cells were counted, significant differences emerged between groups. We propose that studies addressing sperm shape abnormalities should standardize counts to an optimal value of 1000 cells per animal, warranting robust statistical results while providing the best compromise concerning labor time.
Keywords: genotoxicity; lab rodents; sperm abnormalities; standardization; wild rodents.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Bhunya S.P., Pati P.C. Genotoxicity of an inorganic pesticide, copper sulphate in mouse in vivo test system. Cytologia. 1987;52:801–808. doi: 10.1508/cytologia.52.801. - DOI
-
- Lähdetie J. Genotoxicity assays of vinyl acetate and acetaldehyde in male germ cells of mice. Mutat. Res. 1990;6:389–390.
Publication types
Grants and funding
- PTDC/SAU-PUB/26254/2017/Fundação para a Ciência e Tecnologia
- CESAM - UIDP/50017/2020, UIDB/50017/2020, LA/P/0094/2020/Fundação para a Ciência e Tecnologia
- 2020.08694.BD to E.C./Fundação para a Ciência e Tecnologia
- national funds (OE) through FCT (Fundação para a Ciência e a Tecnologia, I.P.) in the scope of the framework contract foreseen in the numbers 4, 5 and 6 of article 23, of the Decree-Law 57/2016, of August 29, changed by Law 57/2017, of July 19, to S.I.G./Fundação para a Ciência e Tecnologia
LinkOut - more resources
Full Text Sources
Miscellaneous

