Structural and Functional Changes in Non-Paraneoplastic Autoimmune Retinopathy
- PMID: 37958272
- PMCID: PMC10649684
- DOI: 10.3390/diagnostics13213376
Structural and Functional Changes in Non-Paraneoplastic Autoimmune Retinopathy
Abstract
Background: To describe longitudinal changes in patients with non-paraneoplastic autoimmune retinopathy (npAIR) by utilizing different diagnostic modalities/tests.
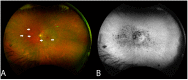
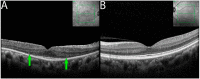
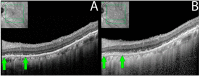
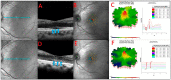
Methods: The index study is a retrospective longitudinal review of sixteen eyes of eight patients from a tertiary care eye hospital diagnosed with npAIR. Multiple diagnostic modalities such as wide-angle fundus photography (WAFP), WA fundus autofluorescence (WAFAF), spectral-domain optical coherence tomography (SD-OCT), Goldmann visual field (GVF) perimetry, microperimetry (MP), electrophysiologic testing, and adaptive optics scanning laser ophthalmoscopy (AOSLO) were reviewed and analyzed.
Results: At the baseline visits, anomalies were detected by multimodal diagnostic tests on all patients. Subjects were followed up for a median duration of 11.5 [3.0-18.7] months. Structural changes at the baseline were detected in 14 of 16 (87.5%) eyes on WAFP and WAFAF and 13 of 16 (81.2%) eyes on SD-OCT. Eight of the ten (80%) eyes that underwent AOSLO imaging depicted structural changes. Functional changes were detected in 14 of 16 (87.5%) eyes on GVF, 15 of 16 (93.7%) eyes on MP, and 11 of 16 (68.7%) eyes on full-field electroretinogram (ff-ERG). Multifocal electroretinogram (mf-ERG) and visual evoked potential (VEP) tests were performed in 14 eyes, of which 12 (85.7%) and 14 (100%) of the eyes demonstrated functional abnormalities, respectively, at baseline. Compared to all the other structural diagnostic tools, AOSLO had a better ability to demonstrate deterioration in retinal microstructures occurring at follow-ups. Functional deterioration at follow-up was detected on GVF in 8 of 10 (80%) eyes, mf-ERG in 4 of 8 (50%) eyes, and MP in 7 of 16 (43.7%) eyes. The ff-ERG and VEP were stable in the majority of cases at follow-up.
Conclusions: The utilization of multimodal imaging/tests in the diagnosing and monitoring of npAIR patients can aid in identifying anomalous changes over time. Analysis of both the anatomical and functional aspects by these devices can be supportive of detecting the changes early in such patients. AOSLO shows promise as it enables the capture of high-resolution images demonstrating quantifiable changes to retinal microstructure.
Keywords: AIR; AOSLO; GVF; Goldmann visual field; adaptive optics; functional changes; microperimetry; non-paraneoplastic autoimmune retinopathy; retina imaging; structural changes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Taylor A.W. Ocular immunosuppressive microenvironment. Chem. Immunol. 1999;73:72–89. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

