CD30 Lateral Flow and Enzyme-Linked Immunosorbent Assays for Detection of BIA-ALCL: A Pilot Study
- PMID: 37958303
- PMCID: PMC10649192
- DOI: 10.3390/cancers15215128
CD30 Lateral Flow and Enzyme-Linked Immunosorbent Assays for Detection of BIA-ALCL: A Pilot Study
Abstract
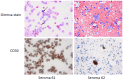
Introduction: Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) commonly presents as a peri-implant effusion (seroma). CD30 (TNFRSF8) is a consistent marker of tumor cells but also can be expressed by activated lymphocytes in benign seromas. Diagnosis of BIA-ALCL currently includes cytology and detection of CD30 by immunohistochemistry or flow cytometry, but these studies require specialized equipment and pathologists' interpretation. We hypothesized that a CD30 lateral flow assay (LFA) could provide a less costly rapid test for soluble CD30 that eventually could be used by non-specialized personnel for point-of-care diagnosis of BIA-ALCL.
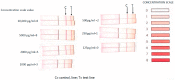
Methods: We performed LFA for CD30 and enzyme-linked immunosorbent assay (ELISA) for 15 patients with pathologically confirmed BIA-ALCL and 10 patients with benign seromas. To determine the dynamic range of CD30 detection by LFA, we added recombinant CD30 protein to universal buffer at seven different concentrations ranging from 125 pg/mL to 10,000 pg/mL. We then performed LFA for CD30 on cryopreserved seromas of 10 patients with pathologically confirmed BIA-ALCL and 10 patients with benign seromas.
Results: Recombinant CD30 protein added to universal buffer produced a distinct test line at concentrations higher than 1000 pg/mL and faint test lines at 250-500 pg/mL. LFA produced a positive test line for all BIA-ALCL seromas undiluted and for 8 of 10 malignant seromas at 1:10 dilution, whereas 3 of 10 benign seromas were positive undiluted but all were negative at 1:10 dilution. Undiluted CD30 LFA had a sensitivity of 100.00%, specificity of 70.00%, positive predictive value of 76.92%, and negative predictive value of 100.00% for BIA-ALCL. When specimens were diluted 1:10, sensitivity was reduced to 80.00% but specificity and positive predictive values increased to 100.00%, while negative predictive value was reduced to 88.33%. When measured by ELISA, CD30 was below 1200 pg/mL in each of six benign seromas, whereas seven BIA-ALCL seromas contained CD30 levels > 2300 pg/mL, in all but one case calculated from dilutions of 1:10 or 1:50.
Conclusions: BIA-ALCL seromas can be distinguished from benign seromas by CD30 ELISA and LFA, but LFA requires less time (<20 min) and can be performed without special equipment by non-specialized personnel, suggesting future point-of-care testing for BIA-ALCL may be feasible.
Keywords: CD30; ELISA; diagnosis; lateral flow assay; lymphoma; screening.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Miranda R.N., Aladily T.N., Prince H.M., Kanagal-Shamanna R., de Jong D., Fayad L.E., Amin M.B., Haideri N., Bhagat G., Brooks G.S., et al. Breast implant-associated anaplastic large-cell lymphoma: Long-term follow-up of 60 patients. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2014;32:114–120. doi: 10.1200/JCO.2013.52.7911. - DOI - PMC - PubMed
-
- de Boer M., van Leeuwen F.E., Hauptmann M., Overbeek L.I.H., de Boer J.P., Hijmering N.J., Sernee A., Klazen C.A.H., Lobbes M.B.I., van der Hulst R.R.W.J., et al. Breast Implants and the Risk of Anaplastic Large-Cell Lymphoma in the Breast. JAMA Oncol. 2018;4:335–341. doi: 10.1001/jamaoncol.2017.4510. - DOI - PMC - PubMed
-
- Cordeiro P.G., Ghione P., Ni A., Hu Q., Ganesan N., Galasso N., Dogan A., Horwitz S.M. Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. J. Plast. Reconstr. Aesthet. Surg. 2020;73:841–846. doi: 10.1016/j.bjps.2019.11.064. - DOI - PMC - PubMed
-
- Campanale A., Di Napoli A., Ventimiglia M., Pileri S., Minella D., Curigliano G., Martelli M., De Vita R., Di Giulio P., Montorsi M., et al. Chest wall infiltration is a critical prognostic factor in breast implant-associated anaplastic large-cell lymphoma affected patients. Eur. J. Cancer. 2021;148:277–286. doi: 10.1016/j.ejca.2021.01.041. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

