The Heterogeneous Impact of Prediagnostic Folate Intake for Fluorouracil-Containing Induction Chemotherapy for Head and Neck Cancer
- PMID: 37958324
- PMCID: PMC10650771
- DOI: 10.3390/cancers15215150
The Heterogeneous Impact of Prediagnostic Folate Intake for Fluorouracil-Containing Induction Chemotherapy for Head and Neck Cancer
Abstract
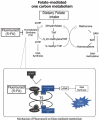
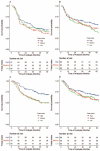
Fluorouracil (FU) exerts its antitumor activity by inhibiting folate-mediated one-carbon metabolism. Evidence that folate may play a role in the carcinogenic process via folate-mediated one-carbon metabolism has given rise to the hypothesis that pre-diagnostic folate intake may induce heterogeneous chemosensitivity to FU-containing induction chemotherapy (IC) in head and neck cancer. To assess this hypothesis, we conducted a cohort study to investigate whether the association between prediagnostic dietary folate intake and cancer survival differed between treatment regimens with and without FU-containing IC in 504 cases of locally advanced (stage III/IV) HNSCC, using an epidemiologic database combined with clinical data. In total, 240 patients were treated with FU-containing IC followed by definitive treatment, and 264 patients were treated with definitive treatment alone. Definitive treatment is defined as (1) the surgical excision of a tumor with clear margins, with or without neck lymph node dissection; or (2) radiotherapy with or without chemotherapy. In the overall cohort of the FU-containing IC group, a higher folate intake was significantly associated with better overall survival (adjusted hazard ratios (HRs) for the highest compared to the lowest folate tertiles (HRT3-T1) = 0.42, 95%CI, 0.25-0.76, Ptrend = 0.003). Conversely, no apparent association between prediagnostic folate intake and survival was observed with definitive treatment alone (HRT3-T1: 0.83, 95%CI, 0.49-1.42, Ptrend = 0.491)). A consideration of the cumulative dose of FU-containing IC showed that the survival impact of prediagnostic folate intake differed statistically significantly by treatment regimen (Pinteraction = 0.012). In conclusion, an association between prediagnostic folate intake and HNSCC survival significantly differed by FU-containing IC. This finding indicates that in the carcinogenic process, folate status causes HNSCC to be heterogenous in terms of one-carbon metabolism.
Keywords: fluorouracil; folate intake; head and neck squamous cell carcinoma; induction chemotherapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Induction chemotherapy followed by concurrent radio-chemotherapy versus concurrent radio-chemotherapy alone as treatment of locally advanced squamous cell carcinoma of the head and neck (HNSCC): A meta-analysis of randomized trials.Radiother Oncol. 2016 Feb;118(2):238-43. doi: 10.1016/j.radonc.2015.10.014. Epub 2015 Nov 14. Radiother Oncol. 2016. PMID: 26589131
-
Induction chemotherapy followed by concurrent chemoradiation in advanced squamous cell carcinoma of the head and neck: final results from a phase II study with docetaxel, cisplatin and 5-fluorouracil with a four-year follow-up.Oral Oncol. 2006 Aug;42(7):675-84. doi: 10.1016/j.oraloncology.2005.12.006. Epub 2006 May 30. Oral Oncol. 2006. PMID: 16731029 Clinical Trial.
-
A phase I study of cabazitaxel in combination with platinum and 5-fluorouracil (PF) in locally advanced squamous cell carcinoma of head and neck (LA-SCCHN).Oral Oncol. 2017 Aug;71:99-104. doi: 10.1016/j.oraloncology.2017.05.008. Epub 2017 Jun 16. Oral Oncol. 2017. PMID: 28688700 Clinical Trial.
-
Induction chemotherapy decreases the rate of distant metastasis in patients with head and neck squamous cell carcinoma but does not improve survival or locoregional control: a meta-analysis.Oral Oncol. 2012 Nov;48(11):1076-84. doi: 10.1016/j.oraloncology.2012.06.014. Epub 2012 Jul 15. Oral Oncol. 2012. PMID: 22800881 Review.
-
The Effect of Induction Chemotherapy Using Docetaxel, Cisplatin, and Fluorouracil on Survival in Locally Advanced Head and Neck Squamous Cell Carcinoma: A Meta-Analysis.Cancer Res Treat. 2016 Jul;48(3):907-16. doi: 10.4143/crt.2015.359. Epub 2015 Nov 17. Cancer Res Treat. 2016. PMID: 26582394 Free PMC article. Review.
References
-
- Cooper J.S., Pajak T.F., Forastiere A.A., Jacobs J., Campbell B.H., Saxman S.B., Kish J.A., Kim H.E., Cmelak A.J., Rotman M., et al. Postoperative concurrent radiotherapy and chemotherapy for high-risk squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 2004;350:1937–1944. doi: 10.1056/NEJMoa032646. - DOI - PubMed
-
- Haddad R., O’Neill A., Rabinowits G., Tishler R., Khuri F., Adkins D., Clark J., Sarlis N., Lorch J., Beitler J.J., et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): A randomised phase 3 trial. Lancet Oncol. 2013;14:257–264. doi: 10.1016/S1470-2045(13)70011-1. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

