Prognostic Markers within the Tumour Microenvironment in Classical Hodgkin Lymphoma
- PMID: 37958391
- PMCID: PMC10649036
- DOI: 10.3390/cancers15215217
Prognostic Markers within the Tumour Microenvironment in Classical Hodgkin Lymphoma
Abstract
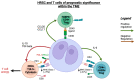
Classical Hodgkin lymphoma (cHL) accounts for 0.4% of all new cancer cases globally. Despite high cure rates with standard treatment, approximately 15% of patients still experience relapsed or refractory (RR) disease, and many of these eventually die from lymphoma-related causes. Exciting new targeted agents such as anti-PD-1 agents and brentuximab vedotin have changed the therapeutic paradigm beyond chemotherapy and radiotherapy alone. Advances in understanding of the molecular biology are providing insights in the context of novel therapies. The signature histology of cHL requires the presence of scant malignant Hodgkin Reed-Sternberg cells (HRSCs) surrounded by a complex immune-rich tumour microenvironment (TME). The TME cellular composition strongly influences outcomes, yet knowledge of the precise characteristics of TME cells and their interactions with HRSCs is evolving. Novel high-throughput technologies and single-cell sequencing allow deeper analyses of the TME and mechanisms elicited by HRSCs to propagate growth and avoid immune response. In this review, we explore the evolution of knowledge on the prognostic role of immune cells within the TME and provide an up-to-date overview of emerging prognostic data on cHL from new technologies that are starting to unwind the complexity of the cHL TME and provide translational insights into how to improve therapy in the clinic.
Keywords: Classical Hodgkin lymphoma; PD-L1; T-cells; immune checkpoint inhibitors; prognostic markers; tumour microenvironment; tumour-associated macrophages.
Conflict of interest statement
Eliza A Hawkes: Consultant or advisory roles at Roche, Merck Sharpe & Dohme, Astra Zeneca, Gilead, Antigene, Novartis, Regeneron, Janssen, Specialised Therapeutics; Research funding: Roche, Bristol Myers Squibb, Merck KgA, Astra Zeneca, and Merck; Colm Keane, Arina Martynchyk, and Rakin Chowdhury—no conflict of interest.
Figures

References
-
- Huang J., Pang W.S., Lok V., Zhang L., Lucero-Prisno D.E., Xu W., Zheng Z.-J., Elcarte E., Withers M., Wong M.C.S., et al. Incidence, mortality, risk factors, and trends for Hodgkin lymphoma: A global data analysis. J. Hematol. Oncol. 2022;15:57. doi: 10.1186/s13045-022-01281-9. - DOI - PMC - PubMed
-
- Carbone P.P., Kaplan H.S., Musshoff K., Smithers D.W., Tubiana M. Report of the Committee on Hodgkin’s Disease Staging Classification. Cancer Res. 1971;31:1860–1861. - PubMed
-
- Cheson B.D., Fisher R.I., Barrington S.F., Cavalli F., Schwartz L.H., Zucca E., Lister T.A. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: The Lugano classification. J. Clin. Oncol. 2014;32:3059–3068. doi: 10.1200/JCO.2013.54.8800. - DOI - PMC - PubMed
-
- Eich H.T., Diehl V., Görgen H., Pabst T., Markova J., Debus J., Ho A., Dörken B., Rank A., Grosu A.L., et al. Intensified chemotherapy and dose-reduced involved-field radiotherapy in patients with early unfavorable Hodgkin’s lymphoma: Final analysis of the German Hodgkin Study Group HD11 trial. J. Clin. Oncol. 2010;28:4199–4206. doi: 10.1200/JCO.2010.29.8018. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

