Tailoring Therapeutic Strategies in Non-Small-Cell Lung Cancer: The Role of Genetic Mutations and Programmed Death Ligand-1 Expression in Survival Outcomes
- PMID: 37958421
- PMCID: PMC10648983
- DOI: 10.3390/cancers15215248
Tailoring Therapeutic Strategies in Non-Small-Cell Lung Cancer: The Role of Genetic Mutations and Programmed Death Ligand-1 Expression in Survival Outcomes
Abstract
Background: This study aims to assess the real-world impact of advancements in first-line systemic therapies for non-small-cell lung cancer (NSCLC), focusing on the role of driver gene mutations and programmed death-ligand 1 (PD-L1) expression levels.
Methods: Conducted across eight medical facilities in Japan, this multicenter, retrospective observational research included 863 patients diagnosed with NSCLC and treated between January 2015 and December 2022. The patients were categorized based on the type of systemic therapy received: cytotoxic agents, molecular targeting agents, immune checkpoint inhibitors, and combination therapies. Comprehensive molecular and immunohistochemical analyses were conducted, and statistical evaluations were performed.
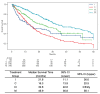
Results: The median overall survival (OS) shows significant variations among treatment groups, with targeted therapies demonstrating the longest OS. This study also revealed that high PD-L1 expression was common in the group treated with immune checkpoint inhibitors. Multivariate analysis was used to identify the type of anticancer drug and the expression of PD-L1 at diagnosis as the impactful variables affecting 5-year OS.
Conclusions: This study underscores the efficacy of targeted therapies and the critical role of comprehensive molecular diagnostics and PD-L1 expression in affecting OS in NSCLC patients, advocating for their integration into routine clinical practice.
Keywords: PD-L1 expression; driver oncogene; immune checkpoint inhibitors; molecular targeting agents; non-small-cell lung cancer; overall survival; personalized medicine; real-world evidence.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
LinkOut - more resources
Full Text Sources
Research Materials

