Whole-Body Imaging for the Primary Staging of Melanomas-A Single-Center Retrospective Study
- PMID: 37958438
- PMCID: PMC10648596
- DOI: 10.3390/cancers15215265
Whole-Body Imaging for the Primary Staging of Melanomas-A Single-Center Retrospective Study
Abstract
Background: Melanoma staging at diagnosis predominantly depends on the tumor thickness. Sentinel lymph node biopsy (SLNB) is a common tool for primary staging. However, for tumors of >4 mm with ulceration, 3D whole-body imaging and, in particular, Fluor-18-Deoxyglucose positron emission tomography combined with computed tomography (18F-FDG-PET/CT), is recommended beforehand. This study aimed to investigate the real-world data of whole-body imaging for initial melanoma staging and its impact on the subsequent diagnostic and therapeutic procedures.
Methods: In this retrospective single-center study, 94 patients receiving 18F-FDG-PET/CT and six patients with whole-body computed tomography (CT) scans were included. The clinical characteristics, imaging results, and histologic parameters of the primary tumors and metastases were analyzed.
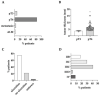
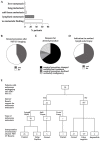
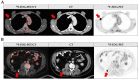
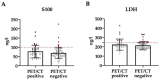
Results: Besides the patients with primary tumors characterized as pT4b (63%), the patients with pT4a tumors and pT3 tumors close to 4 mm in tumor thickness also received initial whole-body imaging. In 42.6% of the patients undergoing 18F-FDG-PET/CT, the imaging results led to a change in the diagnostic or therapeutic procedure following on from this. In 29% of cases, sentinel lymph node biopsy was no longer necessary. The sensitivity and specificity of 18F-FDG-PET/CT were 66.0% and 93.0%, respectively.
Conclusion: Whole-body imaging as a primary diagnostic tool is highly valuable and influences the subsequent diagnostic and therapeutic procedures in a considerable number of patients with a relatively high tumor thickness. It can help avoid the costs and invasiveness of redundant SLNB and simultaneously hasten the staging of patients at the time of diagnosis.
Keywords: PET-CT; diagnosis; imaging; melanoma; staging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
The value of lymph node ultrasound and whole body 18F-FDG PET/CT in stage IIB/C melanoma patients prior to SLNB.Eur J Surg Oncol. 2021 May;47(5):1157-1162. doi: 10.1016/j.ejso.2020.12.007. Epub 2021 Jan 19. Eur J Surg Oncol. 2021. PMID: 33353826
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Lack of clinical impact of 18 F-fluorodeoxyglucose positron emission tomography with simultaneous computed tomography for stage I and II Merkel cell carcinoma with concurrent sentinel lymph node biopsy staging: A single institutional experience from Westmead Hospital, Sydney.Australas J Dermatol. 2017 May;58(2):99-105. doi: 10.1111/ajd.12400. Epub 2015 Oct 13. Australas J Dermatol. 2017. PMID: 26459330
-
PET-CT for staging pT4b melanomas prior to sentinel lymph node biopsy: a 5-year review.Melanoma Res. 2021 Aug 1;31(4):397-401. doi: 10.1097/CMR.0000000000000754. Melanoma Res. 2021. PMID: 34039943 Review.
-
Role of FDG PET-CT in evaluation of locoregional nodal disease for initial staging of breast cancer.World J Clin Oncol. 2014 Dec 10;5(5):982-9. doi: 10.5306/wjco.v5.i5.982. World J Clin Oncol. 2014. PMID: 25493234 Free PMC article. Review.
References
-
- Gershenwald J.E., Scolyer R.A., Hess K.R., Sondak V.K., Long G.V., Ross M.I., Lazar A.J., Faries M.B., Kirkwood J.M., McArthur G.A., et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017;67:472–492. doi: 10.3322/caac.21409. - DOI - PMC - PubMed
-
- Eggermont A.M.M., Blank C.U., Mandalà M., Long G.V., Atkinson V.G., Dalle S., Haydon A.M., Meshcheryakov A., Khattak A., Carlino M.S., et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma (EORTC 1325-MG/KEYNOTE-054): Distant metastasis-free survival results from a double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2021;22:643–654. doi: 10.1016/S1470-2045(21)00065-6. - DOI - PubMed
LinkOut - more resources
Full Text Sources

