Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood
- PMID: 37958529
- PMCID: PMC10648510
- DOI: 10.3390/ijms242115544
Mesenchymal Stromal Cells from Perinatal Tissues as an Alternative for Ex Vivo Expansion of Hematopoietic Progenitor and Stem Cells from Umbilical Cord Blood
Abstract
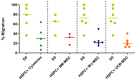
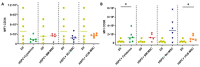
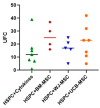
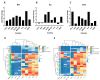
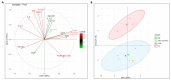
Umbilical cord blood (UCB) serves as a source of hematopoietic stem and progenitor cells (HSPCs) utilized in the regeneration of hematopoietic and immune systems, forming a crucial part of the treatment for various benign and malignant hematological diseases. UCB has been utilized as an alternative HSPC source to bone marrow (BM). Although the use of UCB has extended transplantation access to many individuals, it still encounters significant challenges in selecting a histocompatible UCB unit with an adequate cell dose for a substantial proportion of adults with malignant hematological diseases. Consequently, recent research has focused on developing ex vivo expansion strategies for UCB HSPCs. Our results demonstrate that co-cultures with the investigated mesenchymal stromal cells (MSCs) enable a 10- to 15-fold increase in the cellular dose of UCB HSPCs while partially regulating the proliferation capacity when compared to HSPCs expanded with early acting cytokines. Furthermore, the secretory profile of UCB-derived MSCs closely resembles that of BM-derived MSCs. Moreover, both co-cultures exhibit alterations in cytokine secretion, which could potentially impact HSPC proliferation during the expansion process. This study underscores the fact that UCB-derived MSCs possess a remarkably similar supportive capacity to BM-derived MSCs, implying their potential use as feeder layers in the ex vivo expansion process of HSPCs.
Keywords: hematopoietic stem and progenitor cells ex vivo expansion; hematopoietic stem and progenitor cells transplantation; mesenchymal stromal cells; perinatal tissues; umbilical cord blood.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- MacMillan M.L., Robin M., Harris A.C., DeFor T.E., Martin P.J., Alousi A., Ho V.T., Bolaños-Meade J., Ferrara J.L.M., Jones R., et al. A Refined Risk Score for Acute Graft-versus-Host Disease That Predicts Response to Initial Therapy, Survival, and Transplant-Related Mortality. Biol. Blood Marrow Transpl. 2015;21:761–767. doi: 10.1016/j.bbmt.2015.01.001. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

