Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration
- PMID: 37958598
- PMCID: PMC10648301
- DOI: 10.3390/ijms242115619
Vimentin Localization in the Zebrafish Oral Cavity: A Potential Role in Taste Buds Regeneration
Abstract
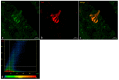
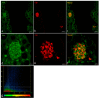
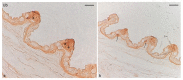
The morphology of the oral cavity of fish is related to their feeding habits. In this context, taste buds are studied for their ability to catch chemical stimuli and their cell renewal capacity. Vimentin RV202 is a protein employed as a marker for mesenchymal cells that can differentiate along different lineages and to self-renew, while Calretinin N-18 is employed as a marker of sensory cells, and ubiquitin is a protein crucial for guiding the fate of stem cells throughout development. In this study, a surface morphology investigation and an immunohistochemical analysis have been conducted. The results of the present study reveal, for the first time, the presence of Vimentin RV202 in a taste bud cell population of zebrafish. Some taste bud cells are just Vimentin RV202-immunoreactive, while in other cells Vimentin RV202 and Calretinin N-18 colocalize. Some taste buds are just reactive to Calretinin N-18. Vimentin RV202-immunoreactive cells have been observed in the connective layer and in the basal portion of the taste buds. The immunoreactivity of ubiquitin was restricted to sensory cells. Further studies are needed to elucidate the role of Vimentin RV202 in the maturation of taste bud cells, its potential involvement in the regeneration of these chemosensory organs, and its eventual synergic work with ubiquitin.
Keywords: Calretinin N-18; SEM; Vimentin RV202; oral cavity; taste buds; zebrafish.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








Similar articles
-
Piezo 1 and Piezo 2 in the Chemosensory Organs of Zebrafish (Danio rerio).Int J Mol Sci. 2024 Jul 5;25(13):7404. doi: 10.3390/ijms25137404. Int J Mol Sci. 2024. PMID: 39000511 Free PMC article.
-
Localization of BDNF and Calretinin in Olfactory Epithelium and Taste Buds of Zebrafish (Danio rerio).Int J Mol Sci. 2022 Apr 23;23(9):4696. doi: 10.3390/ijms23094696. Int J Mol Sci. 2022. PMID: 35563087 Free PMC article.
-
Distribution of α-Gustducin and Vimentin in premature and mature taste buds in chickens.Biochem Biophys Res Commun. 2016 Oct 14;479(2):305-311. doi: 10.1016/j.bbrc.2016.09.064. Epub 2016 Sep 14. Biochem Biophys Res Commun. 2016. PMID: 27639649 Free PMC article.
-
Acid-sensing ion channels and transient-receptor potential ion channels in zebrafish taste buds.Ann Anat. 2016 Sep;207:32-7. doi: 10.1016/j.aanat.2016.06.006. Epub 2016 Aug 8. Ann Anat. 2016. PMID: 27513962 Review.
-
Taste buds as peripheral chemosensory processors.Semin Cell Dev Biol. 2013 Jan;24(1):71-9. doi: 10.1016/j.semcdb.2012.12.002. Epub 2012 Dec 20. Semin Cell Dev Biol. 2013. PMID: 23261954 Free PMC article. Review.
Cited by
-
Localization of Piezo 1 and Piezo 2 in Lateral Line System and Inner Ear of Zebrafish (Danio rerio).Int J Mol Sci. 2024 Aug 24;25(17):9204. doi: 10.3390/ijms25179204. Int J Mol Sci. 2024. PMID: 39273152 Free PMC article.
-
Piezo 1 and Piezo 2 in the Chemosensory Organs of Zebrafish (Danio rerio).Int J Mol Sci. 2024 Jul 5;25(13):7404. doi: 10.3390/ijms25137404. Int J Mol Sci. 2024. PMID: 39000511 Free PMC article.
References
-
- Buddington R.K., Kuz’mina V. Chapter 23—Digestive System. In: Ostrander G.K., editor. The Laboratory Fish. Academic Press; London, UK: 2000. pp. 379–384. - DOI
-
- Abbate F., Guerrera M.C., Levanti M., Laurà R., Aragona M., Mhalhel K., Montalbano G., Germanà A. Morphological characteristics of the blackspot seabream (Pagellus bogaraveo) tongue: A structural and immunohistochemical study. Anat. Histol. Embryol. 2022;51:103–111. doi: 10.1111/ahe.12769. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

