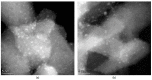
XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation
- PMID: 37958626
- PMCID: PMC10650017
- DOI: 10.3390/ijms242115643
XPS and HR TEM Elucidation of the Diversity of Titania-Supported Single-Site Ir Catalyst Performance in Spin-Selective Propene Hydrogenation
Abstract
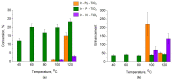
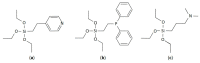
Immobilized [Ir(COD)Cl]2-Linker/TiO2 catalysts with linkers containing Py, P(Ph)2 and N(CH3)2 functional groups were prepared. The catalysts were tested via propene hydrogenation with parahydrogen in a temperature range from 40 °C to 120 °C which was monitored via NMR. The catalytic behavior of [Ir(COD)Cl]2-Linker/TiO2 is explained on the basis of quantitative and qualitative XPS data analysis performed for the catalysts before and after the reaction at 120 °C. It is shown that the temperature dependence of propene conversion and the enhancement of the NMR signal are explained via a combination of the stabilities of both the linker and immobilized [Ir(COD)Cl]2 complex. It is demonstrated that the N(CH3)2-linker is the most stable at the surface of TiO2 under used reaction conditions. As a result, only this sample shows a rise in the enhancement of the NMR signal in the 100-120 °C temperature range.
Keywords: NMR; XPS; heterogeneous catalysis; hydrogenation; single-site catalysts.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Motokura K., Ding S., Usui K., Kong Y. Enhanced Catalysis Based on the Surface Environment of the Silica-Supported Metal Complex. ACS Catal. 2021;11:11985–12018. doi: 10.1021/acscatal.1c03426. - DOI
-
- Skovpin I.V., Sviyazov S.V., Burueva D.B., Kovtunova L.M., Nartova A.V., Kvon R.I., Bukhtiyarov V.I., Koptyug I.V. Nonequilibrium nuclear spin states of ethylene during acetylene hydrogenetion with parahydrogen over immobilized iridium complexes. Dokl. Phys. Chem. 2023. In press .
-
- Kovtunov K.V., Salnikov O.G., Skovpin I.V., Chukanov N.V., Burueva D.B., Koptyug I.V. Catalytic hydrogenation with parahydrogen: A bridge from homogeneous to heterogeneous catalysis. Pure Appl. Chem. 2020;92:1029–1046. doi: 10.1515/pac-2020-0203. - DOI
-
- Skovpin I.V., Kovtunova L.M., Nartova A.V., Kvon R.I., Bukhtiyarov V.I., Koptyug I.V. Anchored complexes of rhodium and iridium in the hydrogenation of alkynes and olefins with parahydrogen. Catal. Sci. Technol. 2022;12:3247–3253. doi: 10.1039/D1CY02258J. - DOI
-
- Lazaro G., Iglesias M., Femandez-Alvarez F.J., Sanz Miguel P.J., Perez-Torrente J.J., Oro L.A. Synthesis of Poly(silyl ether)s by Rhodium(I)–NHC Catalyzed Hydrosilylation: Homogeneous versus Heterogeneous Catalysis. ChemCatChem. 2013;5:1133–1141. doi: 10.1002/cctc.201200309. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

