Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates
- PMID: 37958742
- PMCID: PMC10649860
- DOI: 10.3390/ijms242115756
Tuning the Envelope Structure of Enzyme Nanoreactors for In Vivo Detoxification of Organophosphates
Abstract
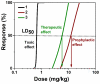
Encapsulated phosphotriesterase nanoreactors show their efficacy in the prophylaxis and post-exposure treatment of poisoning by paraoxon. A new enzyme nanoreactor (E-nRs) containing an evolved multiple mutant (L72C/Y97F/Y99F/W263V/I280T) of Saccharolobus solfataricus phosphotriesterase (PTE) for in vivo detoxification of organophosphorous compounds (OP) was made. A comparison of nanoreactors made of three- and di-block copolymers was carried out. Two types of morphology nanoreactors made of di-block copolymers were prepared and characterized as spherical micelles and polymersomes with sizes of 40 nm and 100 nm, respectively. The polymer concentrations were varied from 0.1 to 0.5% (w/w) and enzyme concentrations were varied from 2.5 to 12.5 μM. In vivo experiments using E-nRs of diameter 106 nm, polydispersity 0.17, zeta-potential -8.3 mV, and loading capacity 15% showed that the detoxification efficacy against paraoxon was improved: the LD50 shift was 23.7xLD50 for prophylaxis and 8xLD50 for post-exposure treatment without behavioral alteration or functional physiological changes up to one month after injection. The pharmacokinetic profiles of i.v.-injected E-nRs made of three- and di-block copolymers were similar to the profiles of the injected free enzyme, suggesting partial enzyme encapsulation. Indeed, ELISA and Western blot analyses showed that animals developed an immune response against the enzyme. However, animals that received several injections did not develop iatrogenic symptoms.
Keywords: enzyme nanoreactor; immune response; organophosphate poisoning; pharmacokinetics; phosphotriesterase; polymersomes; post-exposure treatment; prophylaxis.
Conflict of interest statement
The authors declare the following competing financial interest(s): E.C. and D.D. have filed the patent FR3068989. P.J., D.D. and E.C. report receiving personal fees from Gene&GreenTK during the study. E.C. and D.D. are shareholders in Gene&GreenTK. D.D. is the CEO of Gene&GreenTK. E.C. and D.D. filed the patent FR3068989. The other authors declare no competing financial interest.
Figures






References
-
- Salinas G., Beladi-Mousavi S.M., Kuhn A. Recent progress in enzyme-driven micro/nanoswimmers: From fundamentals to potential applications. Curr. Opin. Electrochem. 2022;32:100887. doi: 10.1016/j.coelec.2021.100887. - DOI
-
- Sun Z., Hou Y. Micro/Nanorobots as Active Delivery Systems for Biomedicine: From Self-Propulsion to Controllable Navigation. Adv. Ther. 2022;5:2100228. doi: 10.1002/adtp.202100228. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

