Age-Related Shift in Cardiac and Metabolic Phenotyping Linked to Inflammatory Cytokines and Antioxidant Status in Mice
- PMID: 37958823
- PMCID: PMC10650425
- DOI: 10.3390/ijms242115841
Age-Related Shift in Cardiac and Metabolic Phenotyping Linked to Inflammatory Cytokines and Antioxidant Status in Mice
Abstract
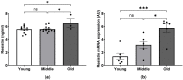
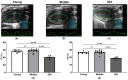
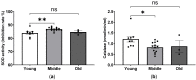
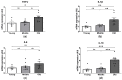
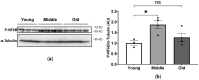
Age-related alterations in cardiac function, metabolic, inflammatory and antioxidant profiles are associated with an increased risk of cardiovascular mortality and morbidity. Here, we examined cardiac and metabolic phenotypes in relation to inflammatory status and antioxidant capacity in young, middle-aged and old mice. Real-time reverse transcription-polymerase chain reactions were performed on myocardium and immunoassays on plasma. Left ventricular (LV) structure and function were assessed by echocardiography using high-frequency ultrasound. Middle-aged mice exhibited an altered metabolic profile and antioxidant capacity compared to young mice, whereas myocardial expression of inflammatory factors (TNFα, IL1β, IL6 and IL10) remained unchanged. In contrast, old mice exhibited increased expression of inflammatory cytokines and plasma levels of resistin compared to young and middle-aged mice (p < 0.05). The pro-inflammatory signature of aged hearts was associated with alterations in glutathione redox homeostasis and elevated contents of 4-hydroxynonenal (4-HNE), a marker of lipid peroxidation and oxidative stress. Furthermore, echocardiographic parameters of LV systolic and diastolic functions were significantly altered in old mice compared to young mice. Taken together, these findings suggest age-related shifts in cardiac phenotype encompass the spectrum of metabo-inflammatory abnormalities and altered redox homeostasis.
Keywords: aging; antioxidant; cardiac function; inflammation and resistin; metabolic status.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Tsao C.W., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., Baker-Smith C.M., Beaton A.Z., Boehme A.K., Buxton A.E., et al. Heart Disease and Stroke Statistics-2023 Update: A Report From the American Heart Association. Circulation. 2023;147:e93–e621. doi: 10.1161/CIR.0000000000001123. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

