Monoterpene Thiols: Synthesis and Modifications for Obtaining Biologically Active Substances
- PMID: 37958865
- PMCID: PMC10649346
- DOI: 10.3390/ijms242115884
Monoterpene Thiols: Synthesis and Modifications for Obtaining Biologically Active Substances
Abstract
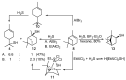
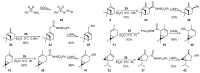
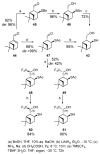
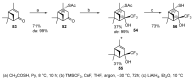
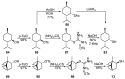
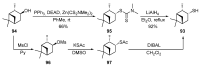
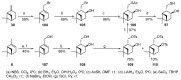
Monoterpene thiols are one of the classes of natural flavors that impart the smell of citrus fruits, grape must and wine, black currants, and guava and are used as flavoring agents in the food and perfume industries. Synthetic monoterpene thiols have found an application in asymmetric synthesis as chiral auxiliaries, derivatizing agents, and ligands for metal complex catalysis and organocatalysts. Since monoterpenes and monoterpenoids are a renewable source, there are emerging trends to use monoterpene thiols as monomers for producing new types of green polymers. Monoterpene thioderivatives are also known to possess antioxidant, anticoagulant, antifungal, and antibacterial activity. The current review covers methods for the synthesis of acyclic, mono-, and bicyclic monoterpene thiols, as well as some investigations related to their usage for the preparation of the compounds with antimicrobial properties.
Keywords: antimicrobial activity; asymmetric synthesis; disulfides; monoterpenoids; sulfenimines; sulfinamides; thiols; thiosulfonates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
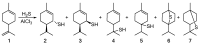

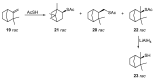
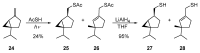
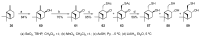

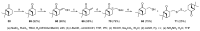




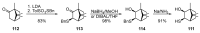




References
-
- Candela K., Fellous R., Joulain D., Faure R. Reduction of Cis- and Trans-1,2-Epithio-p-Menth-8-Ene: Preparation of New Fragrant Terpenoid Thiols. Flavour Fragr. J. 2003;18:52–56. doi: 10.1002/ffj.1153. - DOI
-
- Goeke A. Sulfur-Containing Odorants in Fragrance Chemistry. Sulfur Rep. 2002;23:243–278. doi: 10.1080/01961770208050160. - DOI
-
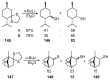
- Martínez-Ramos F., Vargas-Díaz M.E., Chacón-García L., Tamariz J., Joseph-Nathan P., Zepeda L.G. Highly Diastereoselective Nucleophilic Additions Using a Novel Myrtenal-Derived Oxathiane as a Chiral Auxiliary. Tetrahedron Asymmetry. 2001;12:3095–3103. doi: 10.1016/S0957-4166(01)00545-6. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

