Pretreatment Masseter Muscle Volume Predicts Survival in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Chemoradiotherapy
- PMID: 37959329
- PMCID: PMC10648120
- DOI: 10.3390/jcm12216863
Pretreatment Masseter Muscle Volume Predicts Survival in Locally Advanced Nasopharyngeal Carcinoma Patients Treated with Concurrent Chemoradiotherapy
Abstract
Background and purpose: Muscle loss is a significant indicator of cancer cachexia and is associated with a poor prognosis in cancer patients. Given the absence of comparable studies, the current retrospective study sought to examine the correlation between the total masseter muscle volume (TMMV) before treatment and the survival outcomes in locally advanced nasopharyngeal cancer (LA-NPC) patients who received definitive concurrent chemoradiotherapy (CCRT).
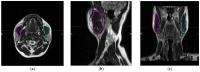
Methods: A three-dimensional segmentation model was used to determine the TMMV for each patient by analyzing pre-CCRT magnetic resonance imaging. The optimal TMMV cutoff values were searched using receiver operating characteristic (ROC) curve analyses. The primary and secondary endpoints were the relationship between the pre-CCRT TMMV measures and overall survival (OS) and progression-free survival (PFS), respectively.
Results: Ninety-seven patients were included in this study. ROC curve analyses revealed 38.0 cc as the optimal TMMV cutoff: ≤38.00 cc (n = 42) and >38.0 cc (n = 55). Comparisons between the two groups showed that the TMMV>38.0 cc group had significantly longer PFS [Not reached (NR) vs. 28; p < 0.01] and OS (NR vs. 71; p < 0.01) times, respectively. The results of the multivariate analysis demonstrated that the T-stage, N-stage, number of concurrent chemotherapy cycles, and TMMV were independent associates of PFS (p < 0.05 for each) and OS (p < 0.05 for each) outcomes, respectively.
Conclusion: The findings of the current retrospective research suggest that pretreatment TMMV is a promising indicator for predicting survival outcomes in LA-NPC patients receiving definitive CCRT.
Keywords: chemoradiotherapy; locally advanced nasopharyngeal cancer; masseter muscle; muscle loss; survival.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
LinkOut - more resources
Full Text Sources

