Progressive Multifocal Leukoencephalopathy in Systemic Lupus Erythematosus: A Consequence of Patient-Intrinsic or -Extrinsic Factors?
- PMID: 37959410
- PMCID: PMC10647998
- DOI: 10.3390/jcm12216945
Progressive Multifocal Leukoencephalopathy in Systemic Lupus Erythematosus: A Consequence of Patient-Intrinsic or -Extrinsic Factors?
Abstract
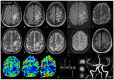
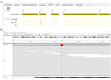
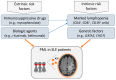
Progressive multifocal leukoencephalopathy (PML) is a severe demyelinating disease of the central nervous system (CNS) caused by reactivation of the polyomavirus JC (JCV) typically in immunocompromised individuals. The risk of PML among rheumatic diseases may be higher for systemic lupus erythematosus (SLE), without, however, a clear association with the type and intensity of background therapy. We present the development and outcome of PML in a 32-year-old female lupus patient under mild immunosuppressive treatment, yet with marked B-cell lymphopenia in the peripheral blood and bone marrow (<1% of total lymphocytes). Despite treatment with the immune checkpoint inhibitor pembrolizumab, the patient showed progressive neurological and brain imaging deterioration and eventually died 15 months after PML diagnosis. To unveil possible underlying genetic liabilities, whole exome sequencing was performed which identified deleterious variants in GATA2 and CDH7 genes, which both have been linked to defective T- and/or B-lymphocyte production. These findings reiterate the possible role of disease-/patient-intrinsic factors, rather than that of drug-induced immunosuppression, in driving immune dysregulation and susceptibility to PML in certain patients with SLE.
Keywords: CDH7; GATA2; immunodeficiency; lymphopenia; polyomavirus.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
LinkOut - more resources
Full Text Sources

