Influence of the Mesoporosity of Hierarchical ZSM-5 in Toluene Alkylation by Methanol
- PMID: 37959471
- PMCID: PMC10649414
- DOI: 10.3390/ma16216872
Influence of the Mesoporosity of Hierarchical ZSM-5 in Toluene Alkylation by Methanol
Abstract
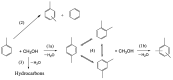
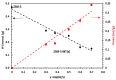
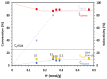
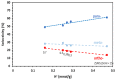
Among the different strategies to design highly shape-selective ZSM-5 to obtain para-xylene through toluene alkylation with methanol, the introduction of mesopores to increase reactant and product diffusion has been proposed but barely studied. In this study, we prepared mesoporous ZSM-5 catalysts, named ZSM5-MT(x), from commercial ZSM-5 (Si/Al = 15), using a two-step micelle-templating procedure with octadecyltrimethylammonium bromide as a surfactant in basic medium (x = NaOH/Si). These materials were used as catalysts for the alkylation of toluene by methanol at a low contact time to avoid thermodynamic equilibrium of the xylene isomers. Compared to the parent ZSM-5, the mesoporous ZSM5-MT(x) catalysts did not improve the para-xylene selectivity, revealing that the strategy of increasing diffusion in the catalyst is not a good strategy to follow. However, ZSM5-MT(0.5) showed less deactivation on stream than the parent ZSM-5. Therefore, introducing mesopores to ZSM-5 could be interesting to explore, combined with another strategy of shape selectivity, such as the passivation of the external surface acidity.
Keywords: alkylation; hierarchical ZSM-5; methanol; para-xylene; toluene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
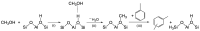



References
-
- Vermeiren W., Gilson J.P. Impact of Zeolites on the Petroleum and Petrochemical Industry. Top Catal. 2009;52:1131–1161. doi: 10.1007/s11244-009-9271-8. - DOI
-
- Martinez C., Corma A. Inorganic molecular sieves: Preparation, modification and industrial application in catalytic processes. Coord. Chem. Rev. 2011;255:1558–1580. doi: 10.1016/j.ccr.2011.03.014. - DOI
-
- Li Y., Yu J. Emerging applications of zeolites in catalysis, separation and host-guest assembly. Nat. Rev. Mater. 2021;6:1156–1174. doi: 10.1038/s41578-021-00347-3. - DOI
-
- Tanabe K., Holderich W. Industrial application of solid acid–base catalysts. Appl. Catal. A Gen. 1999;181:399–434. doi: 10.1016/S0926-860X(98)00397-4. - DOI
-
- Ahn J.H.J. PhD Thesis. Technische Universitat Munchen; Munich, Germany: 2013. Investigation into the Reaction of Toluene Methylation to P-Xylene over Acidic Zeolites.
LinkOut - more resources
Full Text Sources

