A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications
- PMID: 37959643
- PMCID: PMC10650816
- DOI: 10.3390/ma16217046
A Review: Design from Beta Titanium Alloys to Medium-Entropy Alloys for Biomedical Applications
Abstract
β-Ti alloys have long been investigated and applied in the biomedical field due to their exceptional mechanical properties, ductility, and corrosion resistance. Metastable β-Ti alloys have garnered interest in the realm of biomaterials owing to their notably low elastic modulus. Nevertheless, the inherent correlation between a low elastic modulus and relatively reduced strength persists, even in the case of metastable β-Ti alloys. Enhancing the strength of alloys contributes to improving their fatigue resistance, thereby preventing an implant material from failure in clinical usage. Recently, a series of biomedical high-entropy and medium-entropy alloys, composed of biocompatible elements such as Ti, Zr, Nb, Ta, and Mo, have been developed. Leveraging the contributions of the four core effects of high-entropy alloys, both biomedical high-entropy and medium-entropy alloys exhibit excellent mechanical strength, corrosion resistance, and biocompatibility, albeit accompanied by an elevated elastic modulus. To satisfy the demands of biomedical implants, researchers have sought to synthesize the strengths of high-entropy alloys and metastable β-Ti alloys, culminating in the development of metastable high-entropy/medium-entropy alloys that manifest both high strength and a low elastic modulus. Consequently, the design principles for new-generation biomedical medium-entropy alloys and conventional metastable β-Ti alloys can be converged. This review focuses on the design from β-Ti alloys to the novel metastable medium-entropy alloys for biomedical applications.
Keywords: biomedical alloy; high-entropy alloys; medium-entropy alloy; metastable; β-Ti alloys.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








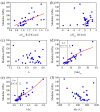
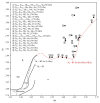
References
-
- Zhang L.C., Chen L.Y. A review on biomedical titanium alloys: Recent progress and prospect. Adv. Eng. Mater. 2019;21:1801215. doi: 10.1002/adem.201801215. - DOI
-
- Zhao J., Xu D., Shahzad M.B., Kang Q., Sun Y., Sun Z., Zhang S., Ren L., Yang C., Yang K. Effect of surface passivation on corrosion resistance and antibacterial properties of Cu-bearing 316L stainless steel. Appl. Surf. Sci. 2016;386:371–380. doi: 10.1016/j.apsusc.2016.06.036. - DOI
-
- Liu Y.P., Gilbert J.L. The effect of simulated inflammatory conditions and pH on fretting corrosion of Co–Cr–Mo alloy surfaces. Wear. 2017;390–391:302–311. doi: 10.1016/j.wear.2017.08.011. - DOI
-
- Liu Y., Chen B. In vivo corrosion of Co–Cr–Mo alloy and biological responses: A review. Mater. Technol. 2017;33:127–134. doi: 10.1080/10667857.2017.1408929. - DOI
-
- Bocchetta P., Chen L.Y., Tardelli J.D.C., Reis A.C., Almeraya-Calderón F., Leo P. Passive layers and corrosion resistance of biomedical Ti–6Al–4V and β-Ti alloys. Coatings. 2021;11:487. doi: 10.3390/coatings11050487. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

