On Prototropy and Bond Length Alternation in Neutral and Ionized Pyrimidine Bases and Their Model Azines in Vacuo
- PMID: 37959699
- PMCID: PMC10648772
- DOI: 10.3390/molecules28217282
On Prototropy and Bond Length Alternation in Neutral and Ionized Pyrimidine Bases and Their Model Azines in Vacuo
Abstract
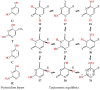
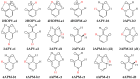
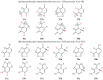
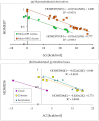
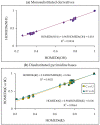
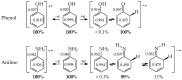
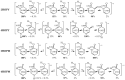
In this review, the complete tautomeric equilibria are derived for disubstituted pyrimidine nucleic acid bases starting from phenol, aniline, and their model compounds-monosubstituted aromatic azines. The differences in tautomeric preferences for isolated (gaseous) neutral pyrimidine bases and their model compounds are discussed in light of different functional groups, their positions within the six-membered ring, electronic effects, and intramolecular interactions. For the discussion of tautomeric preferences and for the analysis of internal effects, recent quantum-chemical results are taken into account and compared to some experimental ones. For each possible tautomer-rotamer of the title compounds, the bond length alternation, measured by means of the harmonic oscillator model of electron delocalization (HOMED) index, is examined. Significant HOMED similarities exist for mono- and disubstituted derivatives. The lack of parallelism between the geometric (HOMED) and energetic (ΔG) parameters for all possible isomers clearly shows that aromaticity is not the main factor that dictates tautomeric preferences for pyrimidine bases, particularly for uracil and thymine. The effects of one-electron loss (positive ionization) and one-electron gain (negative ionization) on prototropy and bond length alternation are also reviewed for pyrimidine bases and their models.
Keywords: bond length alternation; complete tautomeric mixtures; consequences of positive and negative ionization; internal effects; pyrimidine nucleic acid bases; quantum-chemical data in vacuo; tautomeric aromatic azines; tautomeric preferences.
Conflict of interest statement
The author declares no conflict of interest.
Figures









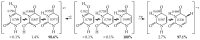

Similar articles
-
Effects of Positive and Negative Ionization on Prototropy in Pyrimidine Bases: An Unusual Case of Isocytosine.J Phys Chem A. 2018 Oct 4;122(39):7863-7879. doi: 10.1021/acs.jpca.8b07539. Epub 2018 Sep 24. J Phys Chem A. 2018. PMID: 30192141
-
Purine tautomeric preferences and bond-length alternation in relation with protonation-deprotonation and alkali metal cationization.J Mol Model. 2020 Apr 4;26(5):93. doi: 10.1007/s00894-020-4343-6. J Mol Model. 2020. PMID: 32248379 Free PMC article.
-
Variations of the tautomeric preferences and π-electron delocalization for the neutral and redox forms of purine when proceeding from the gas phase (DFT) to water (PCM).J Mol Model. 2013 Sep;19(9):3947-60. doi: 10.1007/s00894-013-1926-5. Epub 2013 Jul 7. J Mol Model. 2013. PMID: 23832652 Free PMC article.
-
[Mechanisms of targeted frameshift mutations--insertion formation under error-prone or SOS synthesis of DNA containing CIS-SYN cyncyclobutane thymine dimers].Mol Biol (Mosk). 2014 Jul-Aug;48(4):531-42. Mol Biol (Mosk). 2014. PMID: 25842840 Review. Russian.
-
A model for targeted substitution mutagenesis during SOS replication of double-stranded DNA containing cis-syn cyclobutane thymine dimers.Environ Mol Mutagen. 2006 Dec;47(9):733-45. doi: 10.1002/em.20256. Environ Mol Mutagen. 2006. PMID: 17111422 Review.
Cited by
-
Quantum Chemical Studies on the Prototropic and Acid/Base Equilibria for 2-Aminopyrrole in Vacuo-Role of CH Tautomers in the Design of Strong Brønsted Imino N-Bases.Molecules. 2025 May 9;30(10):2112. doi: 10.3390/molecules30102112. Molecules. 2025. PMID: 40430285 Free PMC article.
References
-
- Pauling L. The Nature of the Chemical Bond. 3rd ed. Cornell University Press; New York, NY, USA: 1960.
-
- Perrin C.L., Agranat I., Bagno A., Braslavsky S.E., Fernandes P.A., Gal J.-F., Lloyd-Jones G.C., Mayr H., Murdoch J.R., Nudelman N.S., et al. Glossary of Terms Used in Physical Organic Chemistry (IUPAC Recommendations 2021) Pure Appl. Chem. 2022;94:353–534. doi: 10.1515/pac-2018-1010. - DOI
-
- Elguero J., Marzin C., Katritzky A.R., Linda P. Academic Press; New York, NY, USA: 1976. The Tautomerism of Heterocycles (Advances in Heterocyclic Chemistry: Supplement 1)
-
- Stanovnik B., Tišler M., Katritzky A.R., Denisko O.V. The Tautomerism of Heterocycles: Substituent Tautomerism of Six-Membered Ring Heterocycles. Adv. Heterocyclic Chem. 2006;91:1–134.
Publication types
LinkOut - more resources
Full Text Sources

