A New Stereoselective Approach to the Substitution of Allyl Hydroxy Group in para-Mentha-1,2-diol in the Search for New Antiparkinsonian Agents
- PMID: 37959723
- PMCID: PMC10650740
- DOI: 10.3390/molecules28217303
A New Stereoselective Approach to the Substitution of Allyl Hydroxy Group in para-Mentha-1,2-diol in the Search for New Antiparkinsonian Agents
Abstract
Two approaches to the synthesis of para-menthene epoxide ((1S,5S,6R)-4) are developed. The first approach includes a reaction between chlorohydrin 7 and NaH in THF. The second involves the formation of epoxide in the reaction of corresponding diacetate 6 with sodium tert-butoxide. One possible mechanism of this reaction is proposed to explain unexpected outcomes in the regio- and stereospecificity of epoxide (1S,5S,6R)-4 formation. The epoxide ring in (1S,5S,6R)-4 is then opened by various S- and O-nucleophiles. This series of reactions allows for the stereoselective synthesis of diverse derivatives of the monoterpenoid Prottremine 1, a compound known for its antiparkinsonian activity, including promising antiparkinsonian properties.
Keywords: Prottremine; epoxide; monoterpene; nucleophilic substitution; synthetic methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


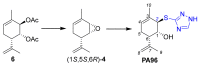

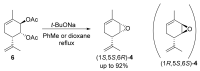

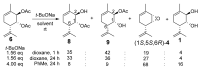






References
Grants and funding
LinkOut - more resources
Full Text Sources

