An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH-Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants
- PMID: 37959754
- PMCID: PMC10648078
- DOI: 10.3390/molecules28217335
An Unusual Rearrangement of Pyrazole Nitrene and Coarctate Ring-Opening/Recyclization Cascade: Formal CH-Acetoxylation and Azide/Amine Conversion without External Oxidants and Reductants
Abstract
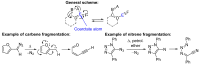
We report an unusual transformation where the transient formation of a nitrene moiety initiates a sequence of steps leading to remote oxidative C-H functionalization (R-CH3 to R-CH2OC(O)R') and the concomitant reduction of the nitrene into an amino group. No external oxidants or reductants are needed for this formal molecular comproportionation. Detected and isolated intermediates and computational analysis suggest that the process occurs with pyrazole ring opening and recyclization.
Keywords: 1H-pyrazole; cyclization; fragmentation; furoxan; nitrene.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. - DOI - PubMed
-
- Boyer J.H. Reduction of Organic Azides to Primary Amines with Lithium Aluminum Hydride. J. Am. Chem. Soc. 1951;73:5865–5866. doi: 10.1021/ja01156a507. - DOI
-
- Rolla F. Sodium borohydride reactions under phase-transfer conditions: Reduction of azides to amines. J. Org. Chem. 1982;47:4327–4329. doi: 10.1021/jo00143a031. - DOI
-
- Ranu B.C., Sarkar A., Chakraborty R. Reduction of Azides with Zinc Borohydride. J. Org. Chem. 1994;59:4114–4116. doi: 10.1021/jo00094a021. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

