Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules
- PMID: 37959771
- PMCID: PMC10649092
- DOI: 10.3390/molecules28217349
Recent Advances in the Synthesis and Antioxidant Activity of Low Molecular Mass Organoselenium Molecules
Abstract
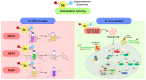
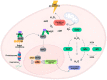
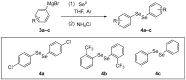
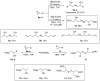
Selenium is an essential trace element in living organisms, and is present in selenoenzymes with antioxidant activity, like glutathione peroxidase (GPx) and thioredoxin reductase (TrxR). The search for small selenium-containing molecules that mimic selenoenzymes is a strong field of research in organic and medicinal chemistry. In this review, we review the synthesis and bioassays of new and known organoselenium compounds with antioxidant activity, covering the last five years. A detailed description of the synthetic procedures and the performed in vitro and in vivo bioassays is presented, highlighting the most active compounds in each series.
Keywords: ABTS; DPPH; FRAP; GPx-like; SOD; antioxidant; catalase; lipid peroxidation; organoselenium.
Conflict of interest statement
M.J.D. is a Director and major shareholder in Seleno Therapeutics plc, which holds patents on selenosugar compounds. The other authors declare no conflict of interest.
Figures


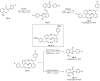

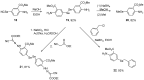
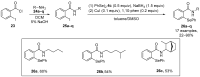
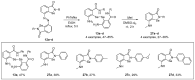
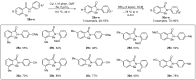

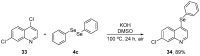
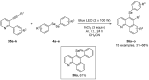
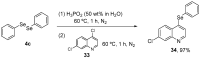
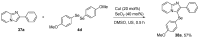
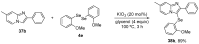



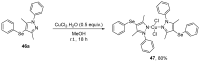
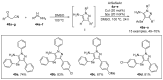
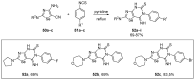
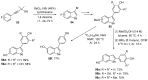
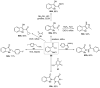

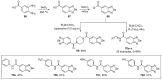

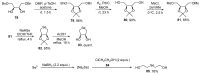
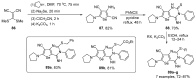
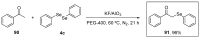
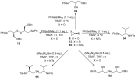
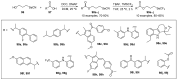
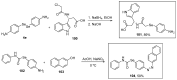
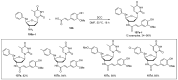

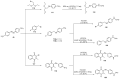
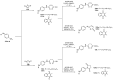
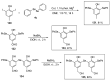
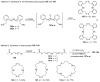


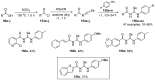



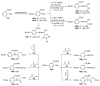
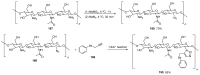
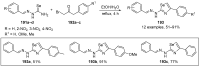
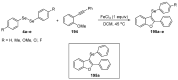
References
-
- Obieziurska-Fabisiak M., Pacuła-Miszewska A.J., Laskowska A., Ścianowski J. Organoselenium compounds as antioxidants. Arkivoc. 2022;5:69–92. doi: 10.24820/ark.5550190.p011.908. - DOI
Publication types
MeSH terms
Substances
Grants and funding
- 422645/2021-4/National Council for Scientific and Technological Development
- 21/2551-0002094-6/Fundação de Amparo à Pesquisa do Estado do Rio Grande do Sul
- 001/Coordenação de Aperfeicoamento de Pessoal de Nível Superior
- CT-INFRA/Financiadora de Estudos e Projetos
- NNF20SA0064214/Novo Nordisk Foundation
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

