Amino Acid Derivatives of Ginsenoside AD-2 Induce HepG2 Cell Apoptosis by Affecting the Cytoskeleton
- PMID: 37959819
- PMCID: PMC10650444
- DOI: 10.3390/molecules28217400
Amino Acid Derivatives of Ginsenoside AD-2 Induce HepG2 Cell Apoptosis by Affecting the Cytoskeleton
Abstract
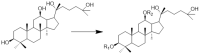
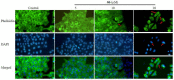
AD-2 (20(R)-dammarane-3β, 12β, 20, 25-tetrol, 25-OH-PPD) was structurally modified to introduce additional amino groups, which can better exert its anti-tumor effects in MCF-7, A549, LoVo, HCT-116, HT -29, and U-87 cell lines. We investigated the cellular activity of 15 different AD-2 amino acid derivatives on HepG2 cells and the possible mechanism of action of the superior derivative 6b. An MTT assay was used to detect the cytotoxicity of the derivatives. Western blotting was used to study the signaling pathways. Flow cytometry was used to detect cell apoptosis and ghost pen peptide staining was used to identify the changes in the cytoskeleton. The AD-2 amino acid derivatives have a better cytotoxic effect on the HepG2 cells than AD-2, which may be achieved by promoting the apoptosis of HepG2 cells and influencing the cytoskeleton. The derivative 6b shows obvious anti-HepG2 cells activity through affecting the expression of apoptotic proteins such as MDM2, P-p53, Bcl-2, Bax, Caspase 3, Cleaved Caspase 3, Caspase 8, and NSD2. According to the above findings, the amino acid derivatives of AD-2 may be developed as HepG2 cytotoxic therapeutic drugs.
Keywords: AD-2; amino acid derivatives; apoptosis; cytotoxic; dammarane triterpene.
Conflict of interest statement
The authors declare no personal relationships or competing financial interests that could have appeared to influence the work reported in this paper.
Figures






References
-
- Gong X., Cui H.T., Bian Y.H., Li Y.T., Wang Y.X., Peng Y.F., Wen W.B., Li K., Wang H.W., Zhang Z.Y., et al. Ethanol extract of Ardisiae Japonicae Herba inhibits hepatoma carcinoma cell proliferation in vitro through regulating lipid metabolism. Chin. Herb. Med. 2021;13:410–415. doi: 10.1016/j.chmed.2021.06.003. - DOI - PMC - PubMed
-
- Li S., Saviano A., Erstad D.J., Hoshida Y., Fuchs B.C., Baumert T., Tanabe K.K. Risk Factors, Pathogenesis, and Strategies for Hepatocellular Carcinoma Prevention: Emphasis on Secondary Prevention and Its Translational Challenges. J. Clin. Med. 2020;9:3817. doi: 10.3390/jcm9123817. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 81703386/National Natural Science Foundation of China
- 2021JH2/10300074/People's Livelihood Plan Project of Department of Science and Technology of Liaoning Province
- LJKMZ20221367/Department of Education of Liaoning Province
- ZQN2018003/Career Development Support Plan for Young and Middle-aged Teachers in Shenyang Pharmaceutical University
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

