Differentiating Dyes: A Spectroscopic Investigation into the Composition of Scarlet Bloodroot (Haemodorum coccineum R.Br.) Rhizome
- PMID: 37959841
- PMCID: PMC10648959
- DOI: 10.3390/molecules28217422
Differentiating Dyes: A Spectroscopic Investigation into the Composition of Scarlet Bloodroot (Haemodorum coccineum R.Br.) Rhizome
Abstract
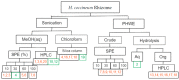
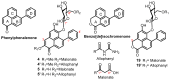
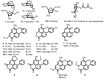
Haemodorum coccineum, commonly known as scarlet bloodroot, is a plant native to New Guinea and the northern most parts of Australia. The highly coloured H. coccineum is used by communities in Larrakia country for dyeing garments and occasionally to treat snake bites. Previous studies into H. coccineum have focused on its taxonomic classification, with this being the first evaluation of the chemical composition of the plant. Haemodoraceae plants are reported to contain phenylphenalenones (PhPs), which are highly conjugated polycyclic oxygenated aromatic hydrocarbons. We report the characterisation of 20 compounds extracted from the rhizome of H. coccineum: four sugars and 16 compounds belonging to the PhP family. The compounds include five aglycones and seven glycosylated compounds, of which four contain malonate esters in their structures. Characterisation of these compounds was achieved through 1D and 2D NMR, MS analysis and comparison to the known phytochemistry of other species from the Haemodorum genus. Preliminary anti-microbial activity of the crude extract shows significant inhibition of the growth of both gram-positive and gram-negative bacteria, but no activity against Candida albicans.
Keywords: Haemodorum coccineum; natural products; phenylphenalenones; traditional medicine; xanthanes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cooke R.G., Segal W. Coloring matters of Australian plants. IV. Haemocorin: A unique glycoside from Haemodorum corymbosum Vahl. Aust. J. Chem. 1955;8:107–113. doi: 10.1071/CH9550107. - DOI
-
- Thomas R. Biosynthesis of the plant phenalenone hemocorin. J. Chem. Soc. D. 1971;14:739–740. doi: 10.1039/c29710000739. - DOI
-
- Thomas R. Biosynthesis of phenalenones. Pure Appl. Chem. 1973;34:515–528. doi: 10.1351/pac197334030515. - DOI
-
- Bick I.R.C., Blackman A.J. Haemodorin. a phenalenone pigment. Aust. J. Chem. 1973;26:1377–1380. doi: 10.1071/CH9731377. - DOI
-
- Edwards J.M. Phenylphenalenones from Wachendorfia species. Phytochemistry. 1974;13:290–291. doi: 10.1016/S0031-9422(00)91320-7. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

