Multifaceted Shape Memory Polymer Technology for Biomedical Application: Combining Self-Softening and Stretchability Properties
- PMID: 37959906
- PMCID: PMC10647621
- DOI: 10.3390/polym15214226
Multifaceted Shape Memory Polymer Technology for Biomedical Application: Combining Self-Softening and Stretchability Properties
Abstract
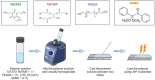
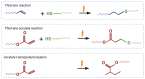
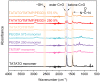
Thiol-ene polymers are a promising class of biomaterials with a wide range of potential applications, including organs-on-a-chip, microfluidics, drug delivery, and wound healing. These polymers offer flexibility, softening, and shape memory properties. However, they often lack the inherent stretchability required for wearable or implantable devices. This study investigated the incorporation of di-acrylate chain extenders to improve the stretchability and conformability of those flexible thiol-ene polymers. Thiol-ene/acrylate polymers were synthesized using 1,3,5-triallyl-1,3,5-triazine-2,4,6(1H,3H,5H)-trione (TATATO), Trimethylolpropanetris (3-mercaptopropionate) (TMTMP), and Polyethylene Glycol Diacrylate (PEGDA) with different molecular weights (Mn 250 and Mn 575). Fourier Transform Infrared (FTIR) spectroscopy confirmed the complete reaction among the monomers. Uniaxial tensile testing demonstrated the softening and stretching capability of the polymers. The Young's Modulus dropped from 1.12 GPa to 260 MPa upon adding 5 wt% PEGDA 575, indicating that the polymer softened. The Young's Modulus was further reduced to 15 MPa under physiologic conditions. The fracture strain, a measure of stretchability, increased from 55% to 92% with the addition of 5 wt% PEGDA 575. A thermomechanical analysis further confirmed that PEGDA could be used to tune the polymer's glass transition temperature (Tg). Moreover, our polymer exhibited shape memory properties. Our results suggested that thiol-ene/acrylate polymers are a promising new class of materials for biomedical applications requiring flexibility, stretchability, and shape memory properties.
Keywords: conformal polymer; flexible polymer; polymer characterization; self-softening polymer; shape memory polymer; stretchable polymer; thiol-ene/acrylate polymer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Lowe A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010;1:17–36. doi: 10.1039/B9PY00216B. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

