Date Palm Extract (Phoenix dactylifera) Encapsulated into Palm Oil Nanolipid Carrier for Prospective Antibacterial Influence
- PMID: 37960029
- PMCID: PMC10648499
- DOI: 10.3390/plants12213670
Date Palm Extract (Phoenix dactylifera) Encapsulated into Palm Oil Nanolipid Carrier for Prospective Antibacterial Influence
Abstract
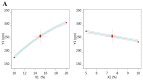
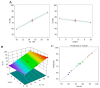
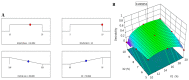
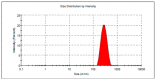
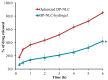
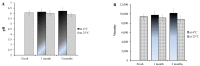
It is worthwhile to note that using natural products today has shown to be an effective strategy for attaining the therapeutic goal with the highest impact and the fewest drawbacks. In Saudi Arabia, date palm (Phoenix dactylifera) is considered the principal fruit owing to its abundance and incredible nutritional benefits in fighting various diseases. The main objective of the study is to exploit the natural products as well as the nanotechnology approach to obtain great benefits in managing disorders. The present investigation focused on using the powder form of date palm extract (DPE) of Khalas cultivar and incorporates it into a nanolipid formulation such as a nanostructured lipid carrier (NLC) prepared with palm oil. Using the quality by design (QbD) methodology, the most optimized formula was chosen based on the number of assigned parameters. For more appropriate topical application, the optimized DP-NLC was combined with a pre-formulated hydrogel base forming the DP-NLC-hydrogel. The developed DP-NLC-hydrogel was evaluated for various physical properties including pH, viscosity, spreadability, and extrudability. Additionally, the in vitro release of the formulation as well as its stability upon storage under two different conditions of room temperature and refrigerator were investigated. Eventually, different bacterial strains were utilized to test the antibacterial efficacy of the developed formulation. The optimized DP-NLC showed proper particle size (266.9 nm) and in vitro release 77.9%. The prepared DP-NLC-hydrogel showed acceptable physical properties for topical formulation, mainly, pH 6.05, viscosity 9410 cP, spreadability 57.6 mm, extrudability 84.5 (g/cm2), and in vitro release 42.4%. Following three months storage under two distinct conditions, the formula exhibited good stability. Finally, the antibacterial activity of the developed DP-NLC-hydrogel was evaluated and proved to be efficient against various bacterial strains.
Keywords: antibacterial; date palm extract; nanolipid carrier; natural products; optimization; palm oil; topical drug delivery.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




Similar articles
-
Nanotechnological prospective for enhancing the antibacterial activity of mupirocin and cinnamon essential oil: a combination therapy.Front Pharmacol. 2024 Nov 11;15:1468374. doi: 10.3389/fphar.2024.1468374. eCollection 2024. Front Pharmacol. 2024. PMID: 39588151 Free PMC article.
-
Date Palm Extract (Phoenix dactylifera) PEGylated Nanoemulsion: Development, Optimization and Cytotoxicity Evaluation.Plants (Basel). 2021 Apr 9;10(4):735. doi: 10.3390/plants10040735. Plants (Basel). 2021. PMID: 33918742 Free PMC article.
-
Tea Tree Oil Nanoemulsion-Based Hydrogel Vehicle for Enhancing Topical Delivery of Neomycin.Life (Basel). 2022 Jul 7;12(7):1011. doi: 10.3390/life12071011. Life (Basel). 2022. PMID: 35888099 Free PMC article.
-
Commercial techniques for preserving date palm (Phoenix dactylifera) fruit quality and safety: A review.Saudi J Biol Sci. 2021 Aug;28(8):4408-4420. doi: 10.1016/j.sjbs.2021.04.035. Epub 2021 Apr 20. Saudi J Biol Sci. 2021. PMID: 34354425 Free PMC article. Review.
-
Date Palm (Phoenix dactylifera L.) Fruit as Potential Antioxidant and Antimicrobial Agents.J Pharm Bioallied Sci. 2019 Jan-Mar;11(1):1-11. doi: 10.4103/jpbs.JPBS_168_18. J Pharm Bioallied Sci. 2019. PMID: 30906133 Free PMC article. Review.
Cited by
-
Investigating topical delivery of erythromycin laden into lipid nanocarrier for enhancing the anti-bacterial activity.Saudi Pharm J. 2024 Sep;32(9):102152. doi: 10.1016/j.jsps.2024.102152. Epub 2024 Jul 28. Saudi Pharm J. 2024. PMID: 39165579 Free PMC article.
-
Virgin Coconut Oil-based Nanostructured Lipid Carrier Improves the Hypolipidemic Effect of Rosuvastatin.Int J Nanomedicine. 2024 Aug 5;19:7945-7961. doi: 10.2147/IJN.S463750. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39130688 Free PMC article.
-
Enhancing the Topical Antibacterial Activity of Fusidic Acid via Embedding into Cinnamon Oil Nano-Lipid Carrier.Gels. 2024 Apr 16;10(4):268. doi: 10.3390/gels10040268. Gels. 2024. PMID: 38667687 Free PMC article.
References
-
- Andrade F., Roca-Melendres M.M., Llaguno M., Hide D., Raurell I., Martell M., Vijayakumar S., Oliva M., Schwartz S., Durán-Lara E.F., et al. Smart and eco-friendly N-isopropylacrylamide and cellulose hydrogels as a safe dual-drug local cancer therapy approach. Carbohydr. Polym. 2022;295:119859. doi: 10.1016/j.carbpol.2022.119859. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

