Anti-Biofilm Activity of Carnosic Acid from Salvia rosmarinus against Methicillin-Resistant Staphylococcus aureus
- PMID: 37960038
- PMCID: PMC10647425
- DOI: 10.3390/plants12213679
Anti-Biofilm Activity of Carnosic Acid from Salvia rosmarinus against Methicillin-Resistant Staphylococcus aureus
Abstract
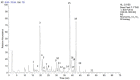
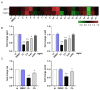
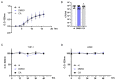
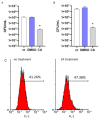
The Salvia rosmarinus "Eretto Liguria" ecotype was studied as a source of valuable bioactive compounds. LC-MS analysis of the methanolic extract underlined the presence of diterpenoids, triterpenoids, polyphenolic acids, and flavonoids. The anti-virulence activity of carnosic acid along with the other most abundant compounds against methicillin-resistant Staphylococcus aureus (MRSA) was evaluated. Only carnosic acid induced a significant reduction in the expression of agrA and rnaIII genes, which encode the key components of quorum sensing (QS), an intracellular signaling mechanism controlling the virulence of MRSA. At a concentration of 0.05 mg/mL, carnosic acid inhibited biofilm formation by MRSA and the expression of genes involved in toxin production and made MRSA more susceptible to intracellular killing, with no toxic effects on eukaryotic cells. Carnosic acid did not affect biofilm formation by Pseudomonas aeruginosa, a human pathogen that often coexists with MRSA in complex infections. The selected ecotype showed a carnosic acid content of 94.3 ± 4.3 mg/g. In silico analysis highlighted that carnosic acid potentially interacts with the S. aureus AgrA response regulator. Our findings suggest that carnosic acid could be an anti-virulence agent against MRSA infections endowed with a species-specific activity useful in multi-microbial infections.
Keywords: MRSA; Salvia rosmarinus; anti-virulence; biofilm; carnosic acid; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
A Novel Aza-Derivative Inhibits agr Quorum Sensing Signaling and Synergizes Methicillin-Resistant Staphylococcus aureus to Clindamycin.Front Microbiol. 2021 Feb 9;12:610859. doi: 10.3389/fmicb.2021.610859. eCollection 2021. Front Microbiol. 2021. PMID: 33633702 Free PMC article.
-
Carnosic acid acts synergistically with gentamicin in killing methicillin-resistant Staphylococcus aureus clinical isolates.Phytomedicine. 2016 Nov 15;23(12):1337-1343. doi: 10.1016/j.phymed.2016.07.010. Epub 2016 Jul 29. Phytomedicine. 2016. PMID: 27765353
-
The effectiveness of anti-biofilm and anti-virulence properties of dihydrocelastrol and dihydrocelastryl diacetate in fighting against methicillin-resistant Staphylococcus aureus.Arch Microbiol. 2017 Oct;199(8):1151-1163. doi: 10.1007/s00203-017-1386-x. Epub 2017 May 9. Arch Microbiol. 2017. PMID: 28487997
-
Methicillin-resistant food-related Staphylococcus aureus: a review of current knowledge and biofilm formation for future studies and applications.Res Microbiol. 2017 Jan;168(1):1-15. doi: 10.1016/j.resmic.2016.08.001. Epub 2016 Aug 17. Res Microbiol. 2017. PMID: 27542729 Review.
-
Correlation Between Biofilm Formation and Antibiotic Resistance in MRSA and MSSA Isolated from Clinical Samples in Iran: A Systematic Review and Meta-Analysis.Microb Drug Resist. 2020 Sep;26(9):1071-1080. doi: 10.1089/mdr.2020.0001. Epub 2020 Mar 10. Microb Drug Resist. 2020. PMID: 32159447
Cited by
-
Cytotoxic Activity of Essential Oils from Middle Eastern Medicinal Plants on Malignant Keratinocytes.Molecules. 2025 Jul 3;30(13):2844. doi: 10.3390/molecules30132844. Molecules. 2025. PMID: 40649358 Free PMC article.
-
Polyphenols Investigation and Antioxidant and Anticholinesterase Activities of Rosmarinus officinalis L. Species from Southwest Romania Flora.Molecules. 2024 Sep 18;29(18):4438. doi: 10.3390/molecules29184438. Molecules. 2024. PMID: 39339433 Free PMC article.
-
Multiple Strategies for the Application of Medicinal Plant-Derived Bioactive Compounds in Controlling Microbial Biofilm and Virulence Properties.Antibiotics (Basel). 2025 May 29;14(6):555. doi: 10.3390/antibiotics14060555. Antibiotics (Basel). 2025. PMID: 40558146 Free PMC article. Review.
References
-
- Drew B.T., González-Gallegos J.G., Xiang C.L., Kriebel R., Drummond C.P., Walked J.B., Sytsma K.J. Salvia united: The greatest good for the greatest number. Taxon. 2017;66:133–145. doi: 10.12705/661.7. - DOI
-
- Roma-Marzio F., Galasso G. New combinations for two hybrids in Salvia subg Rosmarinus (Lamiaceae) Ital. Bot. 2019;7:31–34. doi: 10.3897/italianbotanist.7.34379. - DOI
-
- POWO Plants of the World Online. [(accessed on 6 February 2023)]. Available online: https://powo.science.kew.org.
-
- Ribeiro-Santos R., Carvalho-Costa D., Cavaleiro C., Costa H.S., Albuquerque T.G., Castilho M.C., Ramos F., Melo N.R., Sanches-Silva A. A novel insight on an ancient aromatic plant: The rosemary (Rosmarinus officinalis L.) Trends Food Sci. Technol. 2015;45:355–368. doi: 10.1016/j.tifs.2015.07.015. - DOI
-
- Mulas M., Mulas G. III WOCMAP Congress on Medicinal and Aromatic Plants—Volume 2: Conservation, Cultivation and Sustainable Use of Medicinal and Aromatic Plants. ISHS; Leuven, Belgium: 2005. Cultivar selection from rosemary (Rosmarinus officinalis L.) spontaneous populations in the Mediterranean area; pp. 127–133.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

