Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences
- PMID: 37960049
- PMCID: PMC10648115
- DOI: 10.3390/plants12213692
Impact of Chitosan-Based Foliar Application on the Phytochemical Content and the Antioxidant Activity in Hemp (Cannabis sativa L.) Inflorescences
Abstract
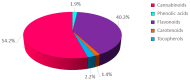
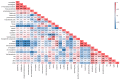
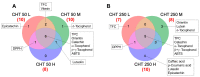
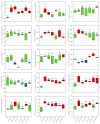
In the present study, the phytochemical content and the antioxidant activity in the inflorescences of the monoecious hemp cultivar Codimono grown in southern Italy were assessed, and their elicitation was induced by foliar spray application of 50 mg/L and 250 mg/L of chitosan (CHT) at three different molecular weights (low, CHT L; medium, CHT M; high CHT H). The analysis of the phytochemical profile confirmed that cannabinoids were the most abundant class (54.2%), followed by flavonoids (40.3%), tocopherols (2.2%), phenolic acids (1.9%), and carotenoids (1.4%). Cannabinoids were represented almost exclusively by cannabidiol, whereas cannabigerol and Δ9-tetrahydrocannabinol were detected at very low levels (the latter was below the legal limit of 0.3%). The most abundant flavonoids were orientin and vitexin, whereas tocopherols were mainly represented by α-tocopherol. The antioxidant activity was found to be positively correlated with flavonoids and tocopherols. Statistical analysis revealed that the CHT treatments significantly affected the phytochemical content and the antioxidant activity of hemp inflorescences. Notably, a significant increase in the total phenolic content (from +36% to +69%), the α-tocopherol (from +45% to +75%) and β+γ-tocopherol (from +35% to +82%) contents, and the ABTS radical scavenging activity (from +12% to +28%) was induced by all the CHT treatments. In addition, treatments with CHT 50 solutions induced an increase in the total flavonoid content (from +12% to +27%), as well as in the vitexin (from +17% to +20%) and orientin (from +20% to +30%) contents. Treatment with CHT 50 L almost always resulted in the greatest increases. Overall, our findings indicated that CHT could be used as a low-cost and environmentally safe elicitor to improve the health benefits and the economic value of hemp inflorescences, thus promoting their employment in the food, pharmaceutical, nutraceutical, and cosmetic supply chains.
Keywords: antioxidant activity; cannabinoids; chitosan; elicitor; industrial hemp; inflorescences; phenolic compounds; tocopherols.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Effect of Genotype, Year, and Their Interaction on the Accumulation of Bioactive Compounds and the Antioxidant Activity in Industrial Hemp (Cannabis sativa L.) Inflorescences.Int J Mol Sci. 2023 May 18;24(10):8969. doi: 10.3390/ijms24108969. Int J Mol Sci. 2023. PMID: 37240314 Free PMC article.
-
Phytochemical Composition and Antioxidant Activity of Various Extracts of Fibre Hemp (Cannabis sativa L.) Cultivated in Lithuania.Molecules. 2023 Jun 22;28(13):4928. doi: 10.3390/molecules28134928. Molecules. 2023. PMID: 37446590 Free PMC article.
-
Industrial Hemp (Cannabis sativa L.) Inflorescences as Novel Food: The Effect of Different Agronomical Practices on Chemical Profile.Foods. 2022 Nov 16;11(22):3658. doi: 10.3390/foods11223658. Foods. 2022. PMID: 36429250 Free PMC article.
-
The Role of Hemp (Cannabis sativa L.) as a Functional Food in Vegetarian Nutrition.Foods. 2023 Sep 20;12(18):3505. doi: 10.3390/foods12183505. Foods. 2023. PMID: 37761214 Free PMC article. Review.
-
Bridging Disciplines: Applications of Forensic Science and Industrial Hemp.Front Microbiol. 2022 Apr 11;13:760374. doi: 10.3389/fmicb.2022.760374. eCollection 2022. Front Microbiol. 2022. PMID: 35479622 Free PMC article. Review.
Cited by
-
Effect of Combining Organic and Inorganic Fertilizers on the Growth of Hemp (Cannabis sativa L.) Plants and the Accumulation of Phytochemicals in Their Inflorescence.Plants (Basel). 2025 May 19;14(10):1519. doi: 10.3390/plants14101519. Plants (Basel). 2025. PMID: 40431084 Free PMC article.
References
-
- Chandra S., Lata H., ElSohly M.A. Cannabis sativa L.-Botany and Biotechnology. Springer International Publishing; Cham, Switzerland: 2017. pp. 1–474.
-
- Ash A.L. Hemp–Production and Utilization. Econ. Bot. 1948;2:159–169. doi: 10.1007/BF02858999. - DOI
-
- Chouvy P.-A. Cannabis cultivation in the world: Heritages, trends and challenges. EchoGéo. 2019;48:50. doi: 10.4000/echogeo.17591. - DOI
-
- Giupponi L., Leoni V., Carrer M., Ceciliani G., Sala S., Panseri S., Pavlovic R., Giorgi A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020;15:1552. doi: 10.4081/ija.2020.1552. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

