Effects of a High-Fat Diet and Docosahexaenoic Acid during Pregnancy on Fatty Acid Composition in the Fetal Livers of Mice
- PMID: 37960348
- PMCID: PMC10649644
- DOI: 10.3390/nu15214696
Effects of a High-Fat Diet and Docosahexaenoic Acid during Pregnancy on Fatty Acid Composition in the Fetal Livers of Mice
Abstract
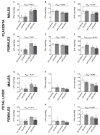
A high-fat diet (HFD) during pregnancy promotes fat accumulation and reduces docosahexaenoic acid (DHA) levels in the liver of the offspring at postnatal ages, which can depend on fetal sex. However, the prenatal mechanisms behind these associations are still unclear. Thus, we analyzed if an HFD alters DHA content and the expression of molecules related to fatty acid (FA) metabolism in the fetal liver. Female C57BL/6 mice were fed a control diet or HFD for 4-6 weeks before pregnancy until the gestational day (GD) 17.5. A subgroup of each diet received DHA (100 mg/Kg) orally from GD 6.5 until 16.5. On GD 17.5, maternal livers, placentas, and livers from male and female fetuses were collected for FA profiling with gas-chromatography and gene expression of molecules related to FA metabolism using qPCR. PPAR-α protein expression was evaluated using Western blot. The gene expression of placental FA transporters was also assessed. An HFD increased eicosapentaenoic acid (EPA) and decreased DHA levels and protein expression of PPAR-α in the fetal livers of both sexes. DHA increased the gene expression of Ppara, Cpt1, and Acsl1 in the livers of female fetuses. Therefore, an HFD reduces DHA levels and PPAR-α, a master regulator of gene expression, in the fetal liver. In turn, the livers of female fetuses seem to be more sensitive to DHA action.
Keywords: docosahexaenoic acid; fetal liver; high-fat diet; maternal liver; placenta; pregnancy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
MeSH terms
Substances
Grants and funding
- FONDECYT 1181798/Agencia Nacional de Investigación y Desarrollo
- FONDECYT 1181774/Agencia Nacional de Investigación y Desarrollo
- FONDECYT 1201483/Agencia Nacional de Investigación y Desarrollo
- FONDECYT 1221098/Agencia Nacional de Investigación y Desarrollo
- CONICYT-PFCHA/Doctorado Nacional/2018-21181253/Agencia Nacional de Investigación y Desarrollo
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

