This is a preprint.
Multiple steps of prion strain adaptation to a new host
- PMID: 37961127
- PMCID: PMC10634783
- DOI: 10.1101/2023.10.24.563743
Multiple steps of prion strain adaptation to a new host
Update in
-
Multiple steps of prion strain adaptation to a new host.Front Neurosci. 2024 Jan 31;18:1329010. doi: 10.3389/fnins.2024.1329010. eCollection 2024. Front Neurosci. 2024. PMID: 38362022 Free PMC article.
Abstract
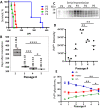
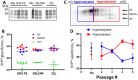
The transmission of prions across species is a critical aspect of their dissemination among mammalian hosts, including humans. This process often necessitates strain adaptation. In this study, we sought to investigate the mechanisms underlying prion adaptation while mitigating biases associated with the history of cross-species transmission of natural prion strains. To achieve this, we utilized the synthetic hamster prion strain S05. Propagation of S05 using mouse PrPC in Protein Misfolding Cyclic Amplification did not immediately overcome the species barrier. This finding underscores the involvement of factors beyond disparities in primary protein structures. Subsequently, we performed five serial passages to stabilize the incubation time to disease in mice. The levels of PrPSc increased with each passage, reaching a maximum at the third passage, and declining thereafter. This suggests that only the initial stage of adaptation is primarily driven by an acceleration in PrPSc replication. During the protracted adaptation to a new host, we observed significant alterations in the glycoform ratio and sialylation status of PrPSc N-glycans. These changes support the notion that qualitative modifications in PrPSc contribute to a more rapid disease progression. Furthermore, consistent with the decline in sialylation, a cue for "eat me" signaling, the newly adapted strain exhibited preferential colocalization with microglia. In contrast to PrPSc dynamics, the intensity of microglia activation continued to increase after the third passage in the new host. In summary, our study elucidates that the adaptation of a prion strain to a new host is a multi-step process driven by several factors.
Keywords: N-linked glycans; cross-species barrier; neurodegenerative diseases; neuroinflammation; prion; prion adaptation; prion diseases; prion strains; reactive astrocytes; reactive microglia; sialylation.
Conflict of interest statement
Conflict of interest. The authors declare that they have no conflicts of interest with the contents of this article.
Figures

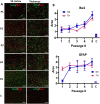
References
-
- Prusiner S. B. (1997) Prion diseases and the BSE crisis. Science 278, 245–251 - PubMed
-
- Legname G., Baskakov I. V., Nguyen H. O. B., Riesner D., Cohen F. E., DeArmond S. J., and Prusiner S. B. (2004) Synthetic mammalian prions. Science 305, 673–676 - PubMed
-
- Collinge J., and Clarke A. R. (2007) A General Model of Prion Strains and Their Pathogenicity. Science 318, 930–936 - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
