This is a preprint.
Plasma-based antigen persistence in the post-acute phase of SARS-CoV-2 infection
- PMID: 37961239
- PMCID: PMC10635183
- DOI: 10.1101/2023.10.24.23297114
Plasma-based antigen persistence in the post-acute phase of SARS-CoV-2 infection
Update in
-
Plasma-based antigen persistence in the post-acute phase of COVID-19.Lancet Infect Dis. 2024 Jun;24(6):e345-e347. doi: 10.1016/S1473-3099(24)00211-1. Epub 2024 Apr 8. Lancet Infect Dis. 2024. PMID: 38604216 Free PMC article. No abstract available.
Abstract
Background: Persistent symptoms among some persons who develop COVID-19 has led to the hypothesis that SARS-CoV-2 may, in some form or location, persist for long periods following acute infection. Several studies have shown data in this regard but are limited by non-representative and small study populations, short duration since acute infection, and lack of a true-negative comparator group to assess assay specificity.
Methods: We evaluated adults with RNA-confirmed COVID-19 at multiple time points following acute infection (pandemic-era participants) and adults with specimens collected prior to 2020 (pre-pandemic era). Using once-thawed plasma, we employed the Simoa® (Quanterix) single molecule array detection platform to measure SARS-CoV-2 spike, S1, and nucleocapsid antigens.
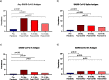
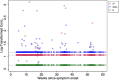
Results: Compared to 250 pre-pandemic participants who had 2% assay positivity, detection of any SARS-CoV-2 antigen was significantly more frequent among 171 pandemic-era participants at three different time periods in the post-acute phase of infection. The absolute difference in SARS-CoV-2 plasma antigen prevalence was +11% (95% CI: +5.0% to +16%) at 3.0-6.0 months post-onset of COVID-19; +8.7% (95% CI: +3.1% to +14%) at 6.1 to 10.0 months; and +5.4% (95% CI: +0.42% to +10%) at 10.1-14.1 months. Hospitalization for acute COVID-19 and, among the non-hospitalized, worse self-reported health during acute COVID-19 were associated with greater post-acute phase antigen detection.
Conclusions: Compared to uninfected persons, there is an excess prevalence of SARS-CoV-2 antigenemia in SARS-CoV-2-infected individuals up to 14 months after acute COVID-19. These findings motivate an urgent research agenda regarding the short-term and long-term clinical manifestations of this viral persistence.
Keywords: COVID-19; Long COVID; antigen; post-acute sequelae of SARS-CoV-2; viral persistence.
Conflict of interest statement
Conflicts of Interest MJP reports consulting for Gilead Sciences and AstraZeneca, outside the submitted work. DRW has a financial interest in Quanterix Corporation, a company that develops an ultra-sensitive digital immunoassay platform. He is an inventor of the Simoa technology, a founder of the company, and also serves on its Board of Directors. Dr. Walt’s interests were reviewed and are managed by Mass General Brigham and Harvard University in accordance with their conflict-of-interest policies.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
