This is a preprint.
Flipped C-Terminal Ends of APOA1 Promote ABCA1-dependent Cholesterol Efflux by Small HDLs
- PMID: 37961344
- PMCID: PMC10635269
- DOI: 10.1101/2023.11.03.23297986
Flipped C-Terminal Ends of APOA1 Promote ABCA1-dependent Cholesterol Efflux by Small HDLs
Update in
-
Flipped C-Terminal Ends of APOA1 Promote ABCA1-Dependent Cholesterol Efflux by Small HDLs.Circulation. 2024 Mar 5;149(10):774-787. doi: 10.1161/CIRCULATIONAHA.123.065959. Epub 2023 Nov 29. Circulation. 2024. PMID: 38018436 Free PMC article.
Abstract
Background: Cholesterol efflux capacity (CEC) predicts cardiovascular disease (CVD) independently of HDL cholesterol (HDL-C) levels. Isolated small HDL particles are potent promoters of macrophage CEC by the ABCA1 pathway, but the underlying mechanisms are unclear.
Methods: We used model system studies of reconstituted HDL and plasma from control and lecithin-cholesterol acyltransferase (LCAT)-deficient subjects to investigate the relationships among the sizes of HDL particles, the structure of APOA1 in the different particles, and the CECs of plasma and isolated HDLs.
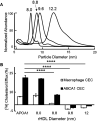
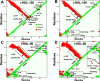
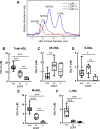
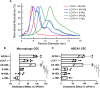
Results: We quantified macrophage and ABCA1 CEC of four distinct sizes of reconstituted HDL (r-HDL). CEC increased as particle size decreased. MS/MS analysis of chemically crosslinked peptides and molecular dynamics simulations of APOA1 (HDL's major protein) indicated that the mobility of that protein's C-terminus was markedly higher and flipped off the surface in the smallest particles. To explore the physiological relevance of the model system studies, we isolated HDL from LCAT-deficient subjects, whose small HDLs-like r-HDLs-are discoidal and composed of APOA1, cholesterol, and phospholipid. Despite their very low plasma levels of HDL particles, these subjects had normal CEC. In both the LCAT-deficient subjects and control subjects, the CEC of isolated extra-small HDL (a mixture of extra-small and small HDL by calibrated ion mobility analysis) was 3-5-fold greater than that of the larger sizes of isolated HDL. Incubating LCAT-deficient plasma and control plasma with human LCAT converted extra-small and small HDL particles into larger particles, and it markedly inhibited CEC.
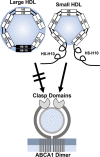
Conclusions: We present a mechanism for the enhanced CEC of small HDLs. In smaller particles, the C-termini of the two antiparallel molecules of APOA1 are flipped off the lipid surface of HDL. This extended conformation allows them to engage with ABCA1. In contrast, the C-termini of larger HDLs are unable to interact productively with ABCA1 because they form a helical bundle that strongly adheres to the lipid on the particle. Enhanced CEC, as seen with the smaller particles, predicts decreased CVD risk. Thus, extra-small and small HDLs may be key mediators and indicators of HDL's cardioprotective effects.
Keywords: ABCA1; chemical crosslinking; cholesterol efflux capacity; molecular dynamics simulation; peptide analysis.
Figures


References
-
- Gordon DJ and Rifkind BM. High-density lipoprotein--the clinical implications of recent studies. N Engl J Med. 1989;321:1311–6. - PubMed
-
- Rader DJ and Hovingh GK. HDL and cardiovascular disease. Lancet. 2014;384:618–625. - PubMed
-
- Rader DJ and Tall AR. The not-so-simple HDL story: Is it time to revise the HDL cholesterol hypothesis? Nat Med. 2012;18:1344–6. - PubMed
-
- Heinecke J. HDL and cardiovascular-disease risk--time for a new approach? N Engl J Med. 2011;364:170–1. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
