This is a preprint.
Decreased fucosylation impacts epithelial integrity and increases risk for COPD
- PMID: 37961411
- PMCID: PMC10635007
- DOI: 10.1101/2023.10.31.564805
Decreased fucosylation impacts epithelial integrity and increases risk for COPD
Abstract
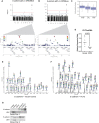
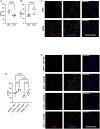
COPD causes significant morbidity and mortality worldwide. Epithelial damage is fundamental to disease pathogenesis, although the mechanisms driving disease remain undefined. Published evidence from a COPD cohort (SPIROMICS) and confirmed in a second cohort (COPDgene) demonstrate a polymorphism in Fucosyltransferese-2 (FUT2) is a trans-pQTL for E-cadherin, which is critical in COPD pathogenesis. We found by MALDI-TOF analysis that FUT2 increased terminal fucosylation of E-cadherin. Using atomic force microscopy, we found that FUT2-dependent fucosylation enhanced E-cadherin-E-cadherin bond strength, mediating the improvement in monolayer integrity. Tracheal epithelial cells from Fut2-/- mice have reduced epithelial integrity, which is recovered with reconstitution of Fut2. Overexpression of FUT2 in COPD derived epithelia rescues barrier function. Fut2-/- mice show increased susceptibility in an elastase model of disease developing both emphysema and fibrosis. We propose this is due to the role of FUT2 in proliferation and cell differentiation. Overexpression of FUT2 significantly increased proliferation. Loss of Fut2 results in accumulation of Spc+ cells suggesting a failure of alveolar type 2 cells to undergo transdifferentiation to alveolar type 1. Using a combination of population data, genetically manipulated mouse models, and patient-derived cells, we present a novel mechanism by which post-translational modifications modulate tissue pathology and serve as a proof of concept for the development of a disease-modifying target in COPD.
Figures






References
-
- The Lancet. UK COPD treatment: failing to progress. Lancet 2018;391:1550. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
