This is a preprint.
Cell-type-resolved somatic mosaicism reveals clonal dynamics of the human forebrain
- PMID: 37961480
- PMCID: PMC10634852
- DOI: 10.1101/2023.10.24.563814
Cell-type-resolved somatic mosaicism reveals clonal dynamics of the human forebrain
Update in
-
Cell-type-resolved mosaicism reveals clonal dynamics of the human forebrain.Nature. 2024 May;629(8011):384-392. doi: 10.1038/s41586-024-07292-5. Epub 2024 Apr 10. Nature. 2024. PMID: 38600385 Free PMC article.
Abstract
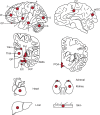
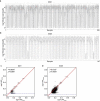
Debate remains around anatomic origins of specific brain cell subtypes and lineage relationships within the human forebrain. Thus, direct observation in the mature human brain is critical for a complete understanding of the structural organization and cellular origins. Here, we utilize brain mosaic variation within specific cell types as distinct indicators for clonal dynamics, denoted as cell-type-specific Mosaic Variant Barcode Analysis. From four hemispheres from two different human neurotypical donors, we identified 287 and 780 mosaic variants (MVs), respectively that were used to deconvolve clonal dynamics. Clonal spread and allelic fractions within the brain reveal that local hippocampal excitatory neurons are more lineage-restricted compared with resident neocortical excitatory neurons or resident basal ganglia GABAergic inhibitory neurons. Furthermore, simultaneous genome-transcriptome analysis at both a cell-type-specific and single-cell level suggests a dorsal neocortical origin for a subgroup of DLX1+ inhibitory neurons that disperse radially from an origin shared with excitatory neurons. Finally, the distribution of MVs across 17 locations within one parietal lobe reveals restrictions of clonal spread in the anterior-posterior axis precedes that of the dorsal-ventral axis for both excitatory and inhibitory neurons. Thus cell-type resolved somatic mosaicism can uncover lineage relationships governing the development of the human forebrain.
Keywords: brain mosaicism; cell type; clonal dynamics; inhibitory neurons; lineage; migration; somatic; whole-genome sequencing.
Conflict of interest statement
Competing interests K.K. is a senior scientist at Bioskryb Genomics Inc. All other authors declare no competing interests.
Figures











References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
