This is a preprint.
Pathological claustrum activity drives aberrant cognitive network processing in human chronic pain
- PMID: 37961503
- PMCID: PMC10635040
- DOI: 10.1101/2023.11.01.564054
Pathological claustrum activity drives aberrant cognitive network processing in human chronic pain
Update in
-
Pathological claustrum activity drives aberrant cognitive network processing in human chronic pain.Curr Biol. 2024 May 6;34(9):1953-1966.e6. doi: 10.1016/j.cub.2024.03.021. Epub 2024 Apr 12. Curr Biol. 2024. PMID: 38614082 Free PMC article.
Abstract
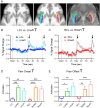
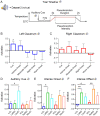
Aberrant cognitive network activity and cognitive deficits are established features of chronic pain. However, the nature of cognitive network alterations associated with chronic pain and their underlying mechanisms require elucidation. Here, we report that the claustrum, a subcortical nucleus implicated in cognitive network modulation, is activated by acute painful stimulation and pain-predictive cues in healthy participants. Moreover, we discover pathological activity of the claustrum and a lateral aspect of the right dorsolateral prefrontal cortex (latDLPFC) in migraine patients. Dynamic causal modeling suggests a directional influence of the claustrum on activity in this latDLPFC region, and diffusion weighted imaging (DWI) verifies their structural connectivity. These findings advance understanding of claustrum function during acute pain and provide evidence of a possible circuit mechanism driving cognitive impairments in chronic pain.
Keywords: Chronic pain; Claustrum; Cognition; Cognitive control; Migraine; Networks; Pain.
Conflict of interest statement
Declaration of Interests: The authors declare no competing interests.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
