This is a preprint.
Spatially resolved DNP-assisted NMR illuminates the conformational ensemble of α-synuclein in intact viable cells
- PMID: 37961511
- PMCID: PMC10634803
- DOI: 10.1101/2023.10.24.563877
Spatially resolved DNP-assisted NMR illuminates the conformational ensemble of α-synuclein in intact viable cells
Update in
-
Spatially resolved DNP-assisted NMR illuminates the conformational ensemble of α-synuclein in intact viable cells.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2500367122. doi: 10.1073/pnas.2500367122. Epub 2025 Jun 4. Proc Natl Acad Sci U S A. 2025. PMID: 40465629
Abstract
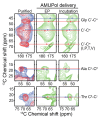
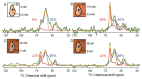
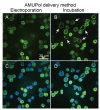
The protein α-syn adopts a wide variety of conformations including an intrinsically disordered monomeric form and an α-helical rich membrane-associated form that is thought to play an important role in cellular membrane processes. However, despite the high affinity of α-syn for membranes, evidence that the α-helical form is adopted inside cells has been indirect. DNP-assisted solid state NMR on frozen cellular samples can report on protein conformations inside cells. Moreover, by controlling the distribution of the DNP polarization agent throughout the cellular biomass, such experiments can provide quantitative information upon the entire structural ensemble or provide information about spatially resolved sub-populations. Using DNP-assisted magic angle spinning (MAS) NMR we establish that purified α-syn in the membrane-associated and intrinsically disordered forms have distinguishable spectra. We then introduced isotopically labeled monomeric α-syn into cells. When the DNP polarization agent is dispersed homogenously throughout the cell, we found that a minority of the α-syn inside cells adopted a highly α-helical rich conformation. When the DNP polarization agent is peripherally localized, we found that the α-helical rich conformation predominates. Thus, we provide direct evidence that α-helix rich conformations of α-syn are adopted near the cellular periphery inside cells under physiological conditions. Moreover, we demonstrate how selectively altering the spatial distribution of the DNP polarization agent can be a powerful tool to observe spatially distinct structural ensembles. This approach paves the way for more nuanced investigations into the conformations that proteins adopt in different areas of the cell.
Keywords: Biophysics and Structural Biology; DNP methods; DNP solid-state NMR; a-synuclein; in-cell NMR.
Figures

Similar articles
-
Spatially resolved DNP-assisted NMR illuminates the conformational ensemble of α-synuclein in intact viable cells.Proc Natl Acad Sci U S A. 2025 Jun 10;122(23):e2500367122. doi: 10.1073/pnas.2500367122. Epub 2025 Jun 4. Proc Natl Acad Sci U S A. 2025. PMID: 40465629
-
DNP-Enhanced MAS NMR: A Tool to Snapshot Conformational Ensembles of α-Synuclein in Different States.Biophys J. 2018 Apr 10;114(7):1614-1623. doi: 10.1016/j.bpj.2018.02.011. Biophys J. 2018. PMID: 29642031 Free PMC article.
-
Structural Context Modulates the Conformational Ensemble of the Intrinsically Disordered Amino Terminus of α-Synuclein.J Am Chem Soc. 2025 Apr 9;147(14):11800-11810. doi: 10.1021/jacs.4c15653. Epub 2025 Mar 27. J Am Chem Soc. 2025. PMID: 40147456
-
Is solid-state NMR enhanced by dynamic nuclear polarization?Solid State Nucl Magn Reson. 2015 Apr-May;66-67:6-20. doi: 10.1016/j.ssnmr.2015.01.003. Epub 2015 Feb 7. Solid State Nucl Magn Reson. 2015. PMID: 25779337 Review.
-
Recent developments in MAS DNP-NMR of materials.Solid State Nucl Magn Reson. 2019 Sep;101:116-143. doi: 10.1016/j.ssnmr.2019.05.009. Epub 2019 May 27. Solid State Nucl Magn Reson. 2019. PMID: 31189121 Review.
References
-
- Theillet F-X, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, et al. Structural disorder of monomeric α-synuclein persists in mammalian cells. Nature. 2016;530(7588):45–50. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
