This is a preprint.
Metabolic similarity and the predictability of microbial community assembly
- PMID: 37961608
- PMCID: PMC10634833
- DOI: 10.1101/2023.10.25.564019
Metabolic similarity and the predictability of microbial community assembly
Abstract
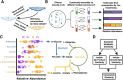
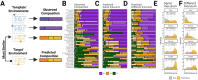
When microbial communities form, their composition is shaped by selective pressures imposed by the environment. Can we predict which communities will assemble under different environmental conditions? Here, we hypothesize that quantitative similarities in metabolic traits across metabolically similar environments lead to predictable similarities in community composition. To that end, we measured the growth rate and by-product profile of a library of proteobacterial strains in a large number of single nutrient environments. We found that growth rates and secretion profiles were positively correlated across environments when the supplied substrate was metabolically similar. By analyzing hundreds of in-vitro communities experimentally assembled in an array of different synthetic environments, we then show that metabolically similar substrates select for taxonomically similar communities. These findings lead us to propose and then validate a comparative approach for quantitatively predicting the effects of novel substrates on the composition of complex microbial consortia.
Conflict of interest statement
Competing interests: The authors declare that no competing interests exist in relation to this manuscript.
Figures



References
-
- Allison S. D., Hanson C. A., and Treseder K. K. 2007. “Nitrogen Fertilization Reduces Diversity and Alters Community Structure of Active Fungi in Boreal Ecosystems.” Soil Biology & Biochemistry. https://www.sciencedirect.com/science/article/pii/S0038071707000685.
-
- Aranda-Díaz Andrés, Willis Lisa, Nguyen Taylor H., Ho Po-Yi, Vila Jean, Thomsen Tani, Chavez Taylor, et al. 2023. “Assembly of Gut-Derived Bacterial Communities Follows ‘early-Bird’ Resource Utilization Dynamics.” bioRxiv. 10.1101/2023.01.13.523996. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
