This is a preprint.
Cytokine polarized, alternatively activated bone marrow neutrophils drive axon regeneration
- PMID: 37961609
- PMCID: PMC10635390
- DOI: 10.21203/rs.3.rs-3491540/v1
Cytokine polarized, alternatively activated bone marrow neutrophils drive axon regeneration
Update in
-
Cytokine polarized, alternatively activated bone marrow neutrophils drive axon regeneration.Nat Immunol. 2024 Jun;25(6):957-968. doi: 10.1038/s41590-024-01836-7. Epub 2024 May 29. Nat Immunol. 2024. PMID: 38811815 Free PMC article.
Abstract
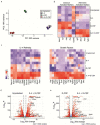
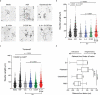
The adult central nervous system (CNS) possesses a limited capacity for self-repair. Severed CNS axons typically fail to regrow. There is an unmet need for treatments designed to enhance neuronal viability, facilitate axon regeneration, and ultimately restore lost neurological functions to individuals affected by traumatic CNS injury, multiple sclerosis, stroke, and other neurological disorders. Here we demonstrate that both mouse and human bone marrow (BM) neutrophils, when polarized with a combination of recombinant interleukin (IL)-4 and granulocyte-colony stimulating factor (G-CSF), upregulate alternative activation markers and produce an array of growth factors, thereby gaining the capacity to promote neurite outgrowth. Moreover, adoptive transfer of IL-4/G-CSF polarized BM neutrophils into experimental models of CNS injury triggered substantial axon regeneration within the optic nerve and spinal cord. These findings have far-reaching implications for the future development of autologous myeloid cell-based therapies that may bring us closer to effective solutions for reversing CNS damage.
Conflict of interest statement
Competing interests B.M.S., A.R.S. and A.D.J. are investors of a patent licensed by the Ohio State University for the use of engineered cells in the treatment of neurological disorders.
Figures




References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

