This is a preprint.
Photoacoustic Imaging for Non-Invasive Assessment of Physiological Biomarkers of Intestinal Injury in Experimental Necrotizing Enterocolitis
- PMID: 37961632
- PMCID: PMC10634697
- DOI: 10.1101/2023.10.20.563296
Photoacoustic Imaging for Non-Invasive Assessment of Physiological Biomarkers of Intestinal Injury in Experimental Necrotizing Enterocolitis
Update in
-
Photoacoustic imaging for non-invasive assessment of biomarkers of intestinal injury in experimental necrotizing enterocolitis.Pediatr Res. 2025 Jan;97(1):169-177. doi: 10.1038/s41390-024-03358-2. Epub 2024 Jun 24. Pediatr Res. 2025. PMID: 38914761 Free PMC article.
Abstract
Background: Necrotizing enterocolitis (NEC) is an often-lethal disease of the premature infants' intestinal tract that is exacerbated by significant difficulties in early and accurate diagnosis. In NEC disease, the intestine often exhibits hypoperfusion and dysmotility, which contributes to advanced disease pathogenesis. However, these physiological features cannot be accurately and quantitively assessed within the current constraints of imaging modalities frequently used in the clinic (plain film X-ray and ultrasound). We have previously demonstrated the ability of photoacoustic imaging (PAI) to non-invasively and quantitively assess intestinal tissue oxygenation and motility in a healthy neonatal rat model. As a first-in-disease application, we evaluated NEC pathogenesis using PAI to assess intestinal health biomarkers in a preclinical neonatal rat experimental model of NEC.
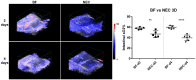
Methods: NEC was induced in neonatal rat pups from birth to 4 days old via hypertonic formula feeding, full-body hypoxic stress, and lipopolysaccharide administration to mimic bacterial colonization. Healthy breastfed (BF) controls and NEC rat pups were imaged at 2- and 4-days old. Intestinal tissue oxygen saturation was measured with PAI imaging for oxy- and deoxyhemoglobin levels. To measure intestinal motility, ultrasound and co-registered PAI cine recordings were used to capture intestinal peristalsis motion and contrast agent (indocyanine green) transit within the intestinal lumen. Additionally, both midplane two-dimensional and volumetric three-dimensional imaging acquisitions were assessed for oxygenation and motility.
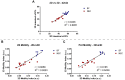
Results: NEC pups showed a significant decrease of intestinal tissue oxygenation as compared to healthy BF controls at both ages (2-days old: 55.90% +/- 3.77% vs 44.12% +/- 7.18%; 4-days old: 56.13% +/- 3.52% vs 38.86% +/- 8.33%). Intestinal motility, assessed using a computational intestinal deformation analysis, demonstrated a significant reduction in the intestinal motility index in both early (2-day) and established (4-day) NEC. Extensive NEC damage was confirmed with histology and dysmotility was confirmed by small intestinal transit assay.
Conclusions: This study presents PAI as a successful emerging diagnostic imaging modality for both intestinal tissue oxygenation and intestinal motility disease hallmarks in a rat NEC model. PAI presents enormous significance and potential for fundamentally changing current clinical paradigms for detecting and monitoring intestinal pathologies in the premature infant.
Keywords: imaging; intestinal motility; intestine; necrotizing enterocolitis; premature infant; tissue oxygenation.
Conflict of interest statement
Competing interests The authors have no competing interests to declare.
Figures




References
-
- Cuna AC, Lee JC, Robinson AL, Allen NH, Foley JE, Chan SS. Bowel Ultrasound for the Diagnosis of Necrotizing Enterocolitis: A Meta-analysis. Ultrasound Q 2018;34:113–8. - PubMed
-
- Raval MV, Moss RL. Current concepts in the surgical approach to necrotizing enterocolitis. Pathophysiology 2014;21:105–10. - PubMed
-
- Epelman M, Daneman A, Navarro OM, Morag I, Moore AM, Kim JH, et al. Necrotizing enterocolitis: review of state-of-the-art imaging findings with pathologic correlation. Radiographics 2007;27:285–305. - PubMed
-
- Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, Ricketts RR, et al. Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 2005;241:984–9; discussion 9–94. - PMC - PubMed
-
- Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–91. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
