This is a preprint.
CXCL12 drives natural variation in coronary artery anatomy across diverse populations
- PMID: 37961706
- PMCID: PMC10635223
- DOI: 10.1101/2023.10.27.23297507
CXCL12 drives natural variation in coronary artery anatomy across diverse populations
Update in
-
CXCL12 drives natural variation in coronary artery anatomy across diverse populations.Cell. 2025 Apr 3;188(7):1784-1806.e22. doi: 10.1016/j.cell.2025.02.005. Epub 2025 Mar 5. Cell. 2025. PMID: 40049164
Abstract
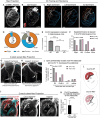
To efficiently distribute blood flow to cardiac muscle, the coronary artery tree must follow a specific branching pattern over the heart. How this pattern arises in humans is unknown due to the limitations of studying human heart development. Here, we leveraged a natural variation of coronary artery anatomy, known as coronary dominance, in genetic association studies to identify the first known driver of human coronary developmental patterning. Coronary dominance refers to whether the right, left, or both coronary arteries branch over the posterior left ventricle, but whether this variability is heritable and how it would be genetically regulated was completely unknown. By conducting the first large-scale, multi-ancestry genome-wide association study (GWAS) of coronary dominance in 61,043 participants of the VA Million Veteran Program, we observed moderate heritability (27.7%) with ten loci reaching genome wide significance. An exceptionally strong association mapped DNA variants to a non-coding region near the chemokine CXCL12 in both European and African ancestries, which overlapped with variants associated with coronary artery disease. Genomic analyses predicted these variants to impact CXCL12 levels, and imaging revealed dominance to develop during fetal life coincident with CXCL12 expression. Reducing Cxcl12 in mice to model the human genetics altered septal artery dominance patterns and caused coronary branches to develop away from Cxcl12 expression domains. Cxcl12 heterozygosity did not compromise overall artery coverage as seen with full deletion, but instead changed artery patterning, reminiscent of the human scenario. Together, our data support CXCL12 as a critical determinant of human coronary artery growth and patterning and lay a foundation for the utilization of developmental pathways to guide future precision 'medical revascularization' therapeutics.
Conflict of interest statement
Competing interests: A.K. is on the scientific advisory board of SerImmune, TensorBio and OpenTargets, and a consultant with Arcadia Science and Inari Agriculture. A.K. was a scientific co-founder of RavelBio, a paid consultant with Illumina, was on the SAB of PatchBio and owns shares in DeepGenomics, Immunai, Freenome, and Illumina. All other authors declare they have no competing interests.
Figures




References
-
- Baroldi G., Scomazzoni G., and United States. Surgeon-General’s, O. (1967). Coronary circulation in the normal and the pathologic heart (Office of the Surgeon General, Dept. of the Army; for sale by the Superintendent of Documents, U.S. Govt. Print. Off.).
-
- Libby P., Bonow R.O., Mann D.L., Tomaselli G.F., Bhattt D.L., and Solomon S. (2021). Braunwald’s heart disease : a textbook of cardiovascular medicine, 12. Edition (Elsevier; ).
-
- Martin S.S., Aday A.W., Almarzooq Z.I., Anderson C.A.M., Arora P., Avery C.L., Baker-Smith C.M., Barone Gibbs B., Beaton A.Z., Boehme A.K., et al. (2024). 2024 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation 149, e347–e913. 10.1161/CIR.0000000000001209. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
