This is a preprint.
Rapid nongenomic estrogen signaling controls alcohol drinking behavior
- PMID: 37961707
- PMCID: PMC10635092
- DOI: 10.1101/2023.11.02.565358
Rapid nongenomic estrogen signaling controls alcohol drinking behavior
Update in
-
Rapid nongenomic estrogen signaling controls alcohol drinking behavior in mice.Nat Commun. 2024 Dec 30;15(1):10725. doi: 10.1038/s41467-024-54737-6. Nat Commun. 2024. PMID: 39737915 Free PMC article.
Abstract
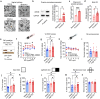
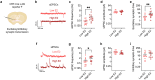
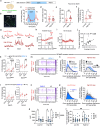
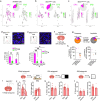
Ovarian-derived estrogen is a key modulator of numerous physiological processes via genomic and nongenomic mechanisms, including signaling non-canonically at membrane-associated estrogen receptors in the brain to rapidly regulate neuronal function. However, the mechanisms mediating estrogen regulation of behaviors such as alcohol consumption remain unclear. Early alcohol drinking confers greater risk for alcohol use disorder in women than men, and binge alcohol drinking is correlated with high circulating estrogen levels, but a causal role for estrogen signaling in driving alcohol drinking in gonadally-intact animals has not been established. We found that female mice displayed greater binge alcohol drinking and reduced avoidance behavior when circulating estrogen was high during the proestrus phase of the estrous cycle than when it was low, contributing to sex differences in these behaviors. The pro-drinking, but not anxiolytic, effect of high endogenous estrogen state occurred via rapid estrogen signaling at membrane-associated estrogen receptor alpha in the bed nucleus of the stria terminalis, which promoted synaptic excitation of corticotropin-releasing factor neurons and facilitated their activity during alcohol drinking behavior. This study is the first to demonstrate a rapid, nongenomic signaling mechanism for ovarian-derived estrogen signaling in the brain controlling behavior in gonadally intact females, and it establishes a causal role for estrogen in an intact hormonal context for driving alcohol consumption that contributes to known sex differences in this behavior.
Conflict of interest statement
Competing interests Authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
